In the allosteric inhibition or non-competitive inhibition Inhibitors bind to the allosteric center of an enzyme and in this way reduce its activity. The binding results in a change in conformation which partially or completely blocks the function of the enzyme. Allosteric inhibition is being considered as a therapy for cancer.
What is Allosteric Inhibition?
.jpg)
Inhibition is used in medicine to describe a slowdown, delay or blockage of biological processes. The action can come to a standstill due to the inhibition. In biochemistry, an inhibition usually corresponds to an enzyme inhibition. This type of inhibition can be either competitive or non-competitive. The non-competitive inhibition is also called allosteric inhibition.
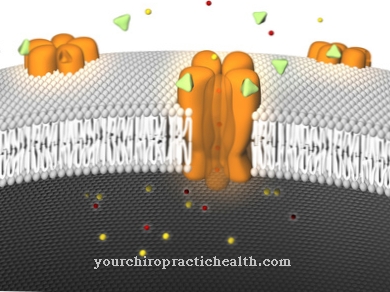
With this type of inhibition, the aim is to bind the inhibitors outside of the active centers of the processes to be inhibited. The inhibitors used and their bonds have a negative impact on the function of an enzyme involved in the process. The inhibitors used are also known as allosteric effectors and, unlike the competitive inhibition of enzymes, do not accumulate in the active process center, but in other locations of the respective enzyme. They are located at the allosteric center of the enzyme and in this way change its conformation. This change in conformation makes it impossible for the enzyme to bind a substrate to the active site, or at least makes it difficult.
Function & task
Enzymes are essential components of every organism. The body's own substances are involved in all metabolic processes and catalyze most of the biochemical reactions. The cells of the body need certain mechanisms to regulate enzymatic processes in order to influence the specific activity of enzymes.
Enzymes are often activated via modifications and their activity is regulated. The binding to certain substances can also play a role in the regulation of enzyme activities. Binding substances are also called effectors, which, depending on their effect on the enzyme, are called either activators or inhibitors. Activators increase the enzymatic activity and promote the associated reaction. Inhibitors reduce enzymatic activities and inhibit the respective reactions.
Inhibitors in the active center of the enzyme bring about what is known as competitive inhibition and occupy the binding sites of the active center. In the case of non-competitive inhibition, the inhibitor binds to the allosteric center of a certain enzyme and thus brings about a structural change in the active center. As a result of these processes, the enzyme either partially or completely loses its function. The feedback inhibition or end product inhibition is a special form of this type of inhibition. A product of synthetic chains allosterically inhibits an enzyme involved in the synthesis.
All types of allosteric inhibition can be reversed. This process corresponds to removing the allostric effectors. Any non-competitive inhibition is based on the binding of inhibitor I to the allosteric center of enzyme E. This binding does not affect substrate binding. The inhibitor can not only bind to the free enzyme but also to its enzyme-substrate complex, since it does not have to bind in the binding part of an enzyme. The respective substrate also reacts with an enzyme-inhibitor complex analogously. However, a formed enzyme-inhibitor-substrate complex does not split off the resulting product. In individual cases of non-competitive inhibition, the specific behavior of inhibitors can deviate more or less from the normal case.
Illnesses & ailments
Inhibition of enzymatic processes is a vital type of regulation in the human body. They can be disturbed, for example, by genetic defects, especially by mutations. Such mutations can affect various building blocks of the human body that play a role in enzyme inhibition. The consequences of not being inhibited can be varied.
Elevated uric acid levels, for example, may be associated with disorders of enzymatic inhibition. If the uric acid concentration in the blood is increased and not enough is excreted with the urine, the salts are deposited in the joints and can thus promote the formation of gouty nodules. The uric acid crystals cause inflammatory reactions in the inner skin of joints, as they are associated with an acute attack of gout. The increased uric acid can be due to a defect in the allosteric inhibition, which favors an increased biosynthesis of so-called purine nucleotides.
Allosteric inhibitions not only form the basis of various diseases, but are now also used by medicine for therapeutic purposes. For example, the allosteric inhibition of BCR-ABL is a current therapeutic principle for chromosome-positive leukemia. Modern medicine also uses the principle of allosteric inhibition in other areas of cancer therapy. Scientists are currently looking for inhibitors in the context of cancer research. In this context, US research groups discovered the Ral proteins, for example, which appear to be of particular interest for cancer research. However, it is not yet possible to speak of a usable drug. Nonetheless, allosteric, non-competitive inhibition is an area that will help shape the future of cancer therapy.








.jpg)

.jpg)


.jpg)