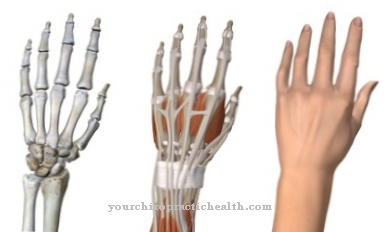
The Inflammatory phase is one of the five phases in secondary fracture healing. It cleaned the breakpoint of bacteria and called immune cells that mediate the reconstruction of the bone. An insufficient inflammatory phase delays the healing of the fracture and can cause pseudarthrosis.
What is the inflammatory phase?

A fracture is a broken bone. Medicine differentiates between indirect and direct fractures. In the case of direct fractures, the fragments are still in contact with each other or at least not more than a millimeter apart. They fit together perfectly and can thus grow together again as part of the primary fracture healing.
In indirect bone fractures, fracture healing is not primary, but secondary. The bone fragments do not fully fit together. The fracture gap between the fragments is more than a millimeter. This gap is bridged and mineralized during the healing process so that the bone forms a whole again. The callus between the fragments is radiologically visible after healing.
The inflammatory phase is one of five phases in secondary fracture healing. The other four phases are the injury phase, the granulation phase, the callus hardening phase and the remodeling phase.
The inflammatory phase begins and will begin immediately after the actual fracture inflammatory phase called. Various immune cells are involved in the phase, especially white blood cells, mast cells and phagocytes, which clear the breakpoint.
Function & task
The inflammatory phase clears the fracture site and the surrounding tissue so that osteoblasts and osteoclasts can work together to rebuild the bone. The previous phase of the fracture only lasts a few seconds. The one to seven day inflammatory phase occurs immediately after the occurrence of a fracture.
With every fracture, blood vessels in the bone and in the adjacent soft tissues are destroyed. The periosteum (the periosteum) and the surrounding muscles are also damaged and bleed into the fracture area. This creates a hematoma.
In addition to the vessels, the canaliculi of the bone fragments are damaged. The interrupted blood supply and the Kanicular lesions separate the osteocytes from the supply and let them die. When they die, the osteocytes release lysosomal enzymes that degenerate the organic matrix and necrotize the ends of the fracture. The resulting tissue debris triggers an immunological inflammation.
Acute phase proteins migrate into the fracture area, for example interleukin-1 or -6. These proteins activate the proteolytic enzyme cascade and thus increase the inflammatory reaction and blood flow. The immigrated platelets give the fracture hematoma stability and release the so-called plateled-derived-growth-factor and transforming-growth-factor-ß. This release calls for reparative cells to act. Granulocytes, macrophages, endothelial cells, lymphocytes, osteoblasts and fibroblasts are mediated.
Many inflammatory mediators allow the endothelial cells to form leukocyte-specific adhesion molecules. The attachment of the leukocytes to the vessel walls is mediated by these molecules. The leukocytes migrate into the wound tissue and fight invading bacteria. They release cytokines that initiate the proliferation and differentiation of hematopoietic cells in the fracture area.
Monocytes also migrate into the fracture area and become macrophages there, which remove cell debris and bacteria and create hypoxic conditions. Angiogen stimulating factors are released. The fracture hematoma in the inflammatory phase is the most important cytokine source in the early healing phase and connects the fracture ends with fibrin threads.
The immunological inflammation prepares the remodeling by gathering all the necessary cells around the fracture site and cleaning them of harmful and disruptive substances. The increased blood supply during this phase reaches six times the normal value after around two weeks, although the inflammatory phase has long since subsided.
Illnesses & ailments
If the inflammatory phase fails to appear after a fracture, then there is probably an immunological impairment. This can have serious consequences. The affected area is not cleaned of bacteria and infections can set in. The healing of the fracture is delayed to a greater or lesser extent. The doctor speaks of delayed wound healing if the fracture site has not ossified after 20 weeks.
In addition to immunological deficiencies, poor blood circulation, for example, can also cause an inadequate inflammatory reaction. Liver diseases, malignancies or vascular diseases, obesity and diabetes mellitus can lead to an ineffective inflammatory phase after fractures.
If the fracture heals only with a long delay due to an immunologically reduced reaction, pseudarthrosis can set in. In addition to chronic swelling, this results in a reduced load capacity of the affected bone. Functional and movement impairments result. In extreme cases, after disturbances in the inflammatory phase, the fracture no longer heals at all or only heals incompletely.
If the fracture site becomes infected, it has serious consequences. The person concerned is weakened and his organism is out of balance. Too weak a defense reaction enables the bacteria to spread. They can affect vital organs via the bloodstream and trigger generalized sepsis, which can be life-threatening. Surgical intervention may be required to prevent this.
In a healthy person of normal weight, however, infection as a result of a fracture is extremely rare. Delay in fracture healing is a much more common phenomenon and is exacerbated by inadequate immobilization of the affected bone.