Hemopexin is a glycoprotein that binds free heme and thereby counteracts oxidative damage in tissue. The liver absorbs the combined heme-hemopexin complex and renders it harmless. Abnormal hemopexin values can occur, for example, in malignant melanoma and hemolytic anemia.
What is Hemopexin?
The protein hemopexin has a strong ability to bind to heme, which occurs in hemoglobin, enzymes and myoglobin. Unbound heme can lead to oxidative stress, which is why the body needs to regulate it. Hemopexin is also under the name Beta-18 glycoprotein known.
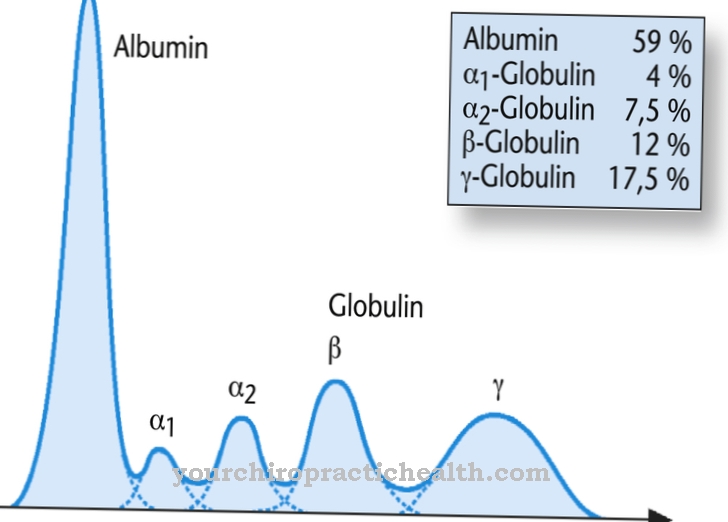
Glycoproteins not only consist of protein, but also contain a carbohydrate content. Hemopexin is also one of the beta globulins, which are a subgroup of globulins. These proteins are found in blood serum and are not soluble in water. Their tasks are, among other things, related to the immune system. In addition, they have numerous specific functions as enzymes, biological transport molecules or regulators of blood properties, for example the pH value. In addition to the beta globulins, there are three other groups in the human body, which biology calls alpha-1, alpha-2 and gamma globulins.
Function, effect & tasks for body and health
When hemopexin encounters a free heme molecule in the blood, the two substances form a bond with each other. In the blood, heme occurs as part of the red blood pigment hemoglobin, which contains iron and is a component of red blood cells (erythrocytes). Their main job is to transport oxygen. In the muscles, hemoglobin corresponds to myoglobin, which can, however, bind oxygen much more strongly.
By forming a haem-haemopexin complex, haemopexin protects the organism from damage caused by free heme, which can cause harmful oxidation of tissue. So-called reactive oxygen species mediate the process. These substances include radicals such as alkoxyl radicals, hydroxyl radicals and peroxyl radicals, but also hydroperoxide, hypochlorite anion, ozone and hydrogen peroxide. Under controlled conditions, the human body uses such reactive oxygen species to fight parasites, bacteria and viruses.
The conversion of energy in the mitochondria also releases small amounts of reactive oxygen species. However, especially in higher concentrations, they lead to oxidative stress, which not only affects proteins and enzymes, but can also affect cytomembrane and genes. If the oxidation is due to free heme, hemopexin can help to limit the damage or to stop the process preventively before major impairments occur.
According to some studies, hemopexin also plays a role in inflammatory processes. However, researchers were able to determine both increased and decreased hemopexin values as correlations. The exact rules that the underlying processes follow have not yet been finally clarified.
Education, occurrence, properties & optimal values
In its primary structure, hemopexin consists of 462 amino acids that are joined together as building blocks in a long chain with the help of peptide bonds. The gene HPX, which is located on the eleventh chromosome in humans, is responsible for the synthesis of the protein.
Like a blueprint, the genetic code defines the sequence of the amino acids within such a chain. Ribosomes use a copy of DNA (the messenger RNA or mRNA) to translate the genetic information into a polypeptide. After the translation is complete, the amino acid chain produced folds and finally takes on the spatial structure of hemopexin. Only in this three-dimensional form is the bioprotein fully functional.
Hemopexin is made in the liver, which also synthesizes most other globulins. In addition, the liver is responsible for producing heme and absorbs the hemopexin when it has bound heme. This process is part of the human body's natural blood purification. The value for hemopexin in the blood serum in healthy people is in the range from 50 to 115 mg per deciliter.
Diseases & Disorders
Abnormal hemopexin levels can occur in the context of various diseases. In the presence of a malignant melanoma, the measured concentration may increase. Malignant melanomas are malignant tumors that grow from the melanocytes.
Melanocytes are skin cells that contain the pigment melanin. This substance is not only responsible for the color of the skin, but also absorbs UV light. Although the absorption is not complete, this mechanism is an important protection against potentially harmful radiation. UV radiation is a component of natural sunlight. Excessive sunbathing and sunburn are therefore among the risk factors associated with the development of melanoma.
Malignant melanoma is also known as black skin cancer because the disease presents as a dark tumor that is brown to black in color on the skin. From a statistical point of view, however, the externally recognizable melanoma disappears in around 20% of those affected. However, this type of cancer often spreads at an early stage and leads to further ulcers in other regions. Treatment options include surgical removal of the tumor and, if necessary, radiation or chemotherapy. If the malignant melanoma has already metastasized, the therapy also takes this into account.
In hemolytic anemia, the level of hemopexin in the blood typically decreases, as this form of anemia is characterized by the breakdown of red blood cells (erythrocytes) that contain heme. The hemopexin binds the released heme and thereby receives a different overall structure with changed properties than unloaded hemopexin. During an analysis, laboratory tests can therefore determine a reduced hemopexin value in the blood serum - in some cases the protein is no longer detectable at all. Pathological hemolysis occurs in the context of various diseases, including sickle and globular cell anemia, rhesus incompatibility or malaria.

.jpg)