Methionine is the only sulfur-containing proteinogenic amino acid besides cysteine. In protein synthesis, L-methionine - its natural and biochemically active form - has a special position because it always represents the first amino acid, the starter substance from which a protein is put together. L-methionine is essential and mainly serves as a supplier of methyl groups (-CH3) for important hormones such as choline, adrenaline, creatine and many more.
What is methionine?
L-methionine (M or Met), the natural and bioactive form of methionine, is an essential, proteinogenic amino acid. Besides cysteine, which is in turn synthesized from methionine, it is the only sulfur-containing amino acid. L-methionine has a special position in the synthesis of proteins because it is always the first amino acid, the starter amino acid, for the structure of every protein.
Methionine is coded on the mRNA (messenger RNA) by the nucleic base triplet adenine-uracil-guanine (AUG), which is also known as the start codon. This means that every mRNA begins with the starter triplet AUG. In order to start the protein synthesis, the tRNA (transport RNA) must first provide L-methionine before the next amino acid can be attached.
Proteins consist of a string of at least 100 proteinogenic amino acids, each of which is linked to one another via a peptide bond. In addition to its role as a component of many proteins, L-methionine is the most important methyl group supplier for the synthesis of hormones such as adrenaline, choline, creatine, histidine and many more. In addition, L-methionine is also a source of sulfur for the synthesis of certain compounds in the body.
Function, effect & tasks
In its biochemically reactive L-form, methionine performs higher-level functions in the body's metabolism as well as specific functions. A superordinate function is to basically form the initial amino acid of a protein.
This means that protein synthesis comes to a standstill if there is not enough L-methionine available in the body. In many cases, however, the methionine is split off and recycled after the protein synthesis has been initiated, so that it is then available again for the next protein synthesis. In some structural proteins in particular, L-methionine is an important component that influences the structural strength of ligaments, tendons and fasciae. The hardness of fingernails and toenails and the strength of the hair also depend on the number of sulfur bridges in the keratin, so that methionine is of great importance here.
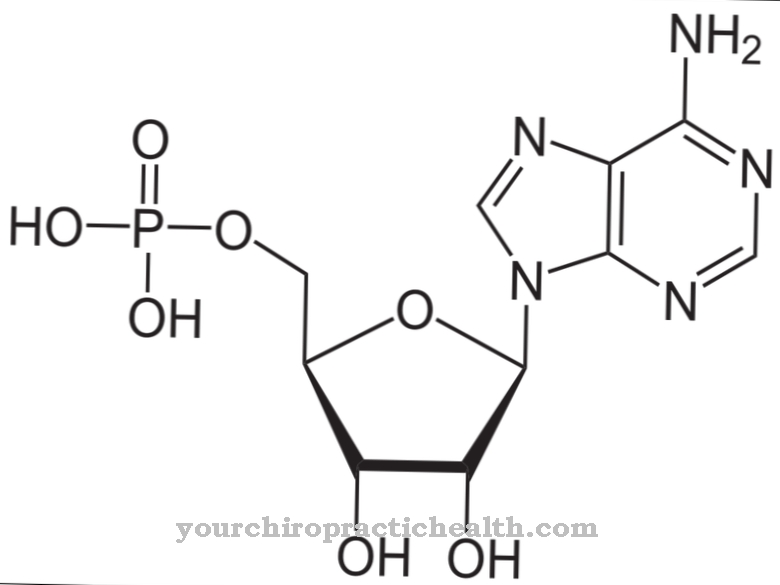
Methione can cross the blood-brain barrier relatively easily and is involved in the formation of the myelin sheaths of the nerves - also in the CNS. Excess methionine that is not directly required can be converted to S-adenosylmethionine (SAM) by adding to ATP (adenosine triphosphate) and act as a methyl group donor (-CH3). After releasing the methyl group, the methionine can be recycled again and is available for further metabolic processes. Excess methionine can be broken down and metabolized to a certain extent over several stages.
Additional methionine doses lead via the physiological degradation process to a deliberate acidification of the urine, which in the case of urinary tract infections inhibits bacterial growth and supports the effect of the administered antibiotics. Calcium phosphate and magnesium ammonium phosphate stones can also dissolve again due to the low pH of the urine.
Education, occurrence, properties & optimal values
Methionine is an essential amino acid, so it has to be supplied from the outside through food. Many foods, both of animal and vegetable origin, contain methionine, but not in free form, but always bound to proteins.
Foods with significant amounts of bound methionine are z. B. raw beef, raw salmon, sesame seeds, dried soybeans and many other foods, including plant-based foods. With over 1,000 mg methionine per 100 g, Brazil nuts even have almost twice as high a content as raw salmon. The proteins are digested in the small intestine. The proteins are largely broken down into smaller pieces (polypeptides) by specialized peptidases and absorbed via the small intestinal villi.
With a balanced diet, it can be assumed that sufficient methionine is consumed. The indications for optimal amounts vary a little. As a reference value, a human requirement of approx. 13 to 16 mg per kilogram of body mass can be assumed. A person of normal weight with a body mass of 75 kg is therefore dependent on a daily intake of methionine in the order of 975 to 1,200 mg.
You can find your medication here
➔ Medicines for muscle weaknessDiseases & Disorders
The essential amino acid methionine serves as a starting material for numerous complex metabolic processes, so that disturbances of certain conversion processes due to the lack of certain enzymes can lead to sometimes serious symptoms. A deficiency in methionine also leads to a deficiency in S-adenosyl methionine (SAM).
The lack of SAM is linked, among other things, to the development of fatty liver disease and the promotion of depression. Some disturbances in the methionine-cysteine metabolism, which are triggered by a deficiency in certain enzymes, lead to an excessive accumulation of the intermediate product homocysteine. The best known cause of homocystinuria, as the accumulation of homocysteine is called, is a genetic defect that causes a deficiency in cystathionine beta synthase.
The excess homocysteine promotes the formation of thromboses and has negative effects on the connective tissue, mainly on the skeleton and the eyes, so that there is a risk of the eye lenses changing position (lens ectopy). Homocystinuria also affects mental processes. When the methionine metabolic disorder leads to a deficiency in cysteine, a deficiency in glutathione and taurine also occurs, which have important protective functions on the nerves. A link between cysteine deficiency and the progression of Alzheimer's disease and Parkinson's disease has been established.












.jpg)