The drug Mitoxantrone belongs to the group of cytostatics. The drug is given against cancer and multiple sclerosis.
What is Mitoxantrone?
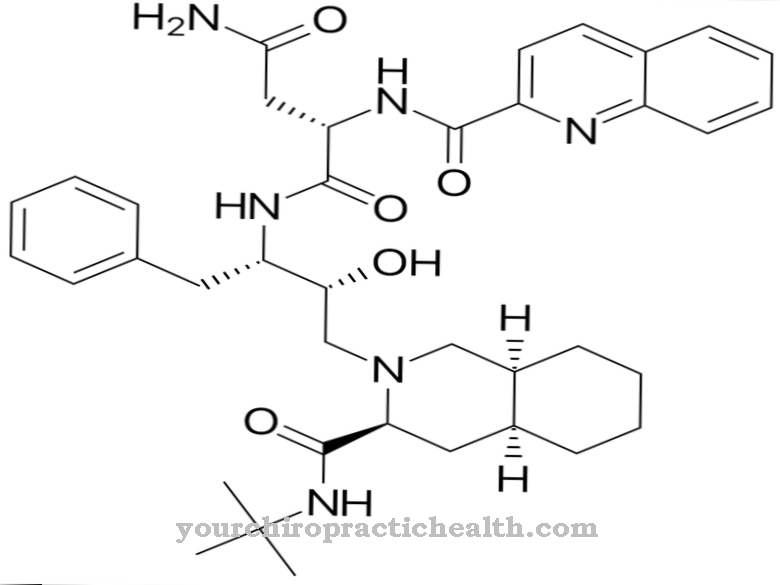
The cytostatic mitoxantrone belongs to the anthracenidione group. It is used to treat malignant cancer and multiple sclerosis. In medicine, the active ingredient also bears the names Mitoxantrone hydrochloride, Mitoxantronum or Mitoxantroni hydrochloridum PhEur.
The drug was approved in the mid-1980s. In Germany, Mitoxantrone is offered as a monopreparation under the trade names Novantron®, Haemato-tron® and Onkotrone®. There are also different generics on the market.
Pharmacological effect
Mitoxantrone has the ability to destroy cancer cells. How this is done has not yet been conclusively clarified. The cytostatic agent damages the DNA (genetic material), which results in the inhibition of DNA synthesis and the subsequent death of the cell. Cancer cells are particularly affected by this process, as they divide faster than healthy cells.
Mitoxantrone is able to develop its effect on the one hand against growing cells and on the other hand against cells in a resting state. Its effect does not depend on the status of cell division. Within the cell cycle, the cytostatic develops in the phase in which the genetic material of the new cell is composed.
The genetic material is damaged by mitoxantrone in different ways. In this way, the cytostatic agent ensures that the build-up of genetic material is inhibited. After they become tangled, the DNA strands break.
In addition, there is an excess of RNA. These are molecules that are responsible for procuring the building blocks of genetic material. In this way, several identical DNA chains are formed, which in turn leads to the death of the cell.
Mitoxantrone can not only have negative effects on cancer cells, but also on bacteria, viruses and parasites. This supports the immune defense of the human body.
In contrast to the anthracyclines, which are often used to treat cancer, mitoxantrone has only a slight tendency to produce free radicals within the tissue. The same applies to the oxidation of blood lipids. These processes ensure that the anthracyclines have a harmful effect on the functions of the human heart. Mitoxantrone has fewer side effects in this regard than the anthracyclines.
Since mitoxantrone is administered intravenously, its bioavailability is 100 percent. There is a protein production of 78 percent. The tissue distribution of the cytostatic agent is very pronounced after intravenous administration. The metabolism of the active ingredient takes place via several cytochrome P450 enzymes. Excretion occurs in the urine and stool.
Medical application & use
Mitoxantrone is suitable for several areas of application. These include various cancers such as advanced breast cancer, which is associated with the formation of metastases (daughter tumors), acute myeloid leukemia (blood cancer), malignant lymphatic cancer (non-Hodgkin syndrome) and advanced prostate cancer that cannot be treated with hormones.
Apart from breast cancer, mitoxantrone is always given together with other cancer drugs. In the case of prostate cancer, a combination with glucocorticoids in low doses can also be used. In this way, the pain is alleviated that can no longer be guaranteed by analgesics or radiation.
Another field of application of mitoxantrone is multiple sclerosis (MS). The cytostatic is used in the treatment of secondary chronic multiple sclerosis. It is also useful for combating relapsing MS, which is progressing rapidly. Studies have shown that mitoxantrone significantly reduces the relapse rate.
Mitoxantrone is always given by intravenous infusion. Ondansetron can also be given against possible nausea, which is also given intravenously.
You can find your medication here
➔ Medicines for paresthesia and circulatory disordersRisks & side effects
The administration of mitoxantrone is not infrequently associated with undesirable side effects. In most cases, hair loss, nausea, vomiting, fever, feelings of weakness and tiredness occur.
There is no menstrual period in women, while in men there is insufficient semen formation.
Other possible side effects are a lack of white blood cells, cardiac arrhythmias, inflammation of the oral mucosa, hypersensitivity reactions, breathing problems, decreased heart pumping, liver disorders, bluish urine, abdominal pain, constipation, loss of appetite and diarrhea.
Heart failure, a heart attack, a lack of blood platelets, gastrointestinal bleeding, chest pain, refusal of food, anemia and a bluish discoloration of veins and nails are less common.
Hypersensitivity to the drug is considered a contraindication for mitoxantrone. Careful weighing of the risks and benefits of treatment is required if the patient has infections, severe kidney or liver dysfunction, severe heart disease, or a lack of all blood cells. The same applies to previous treatment with anthracyclines, as these can affect the heart.
The use of the cytostatic should also be avoided during pregnancy. In addition, consistent contraception is recommended for mitoxantrone therapy. The genetic material can be damaged by the mitoxantrone, which has a negative effect on the child's development. Breastfeeding the baby must also be avoided during treatment with the cytostatic drug. Men are also advised to use contraception as part of mitoxantrone treatment for up to six months after the end of therapy. There is no treatment for children.
When mitoxantrone and other cancer drugs are administered together, there is an increased risk of side effects. In the case of a combination with other cytostatics, blood cancer or bone marrow damage can occur.







.jpg)

.jpg)


.jpg)