The Nicotinamide adenine dinucleotide is an important coenzyme in the context of energy metabolism. It is derived from niacin (vitamin B3, nicotinic acid amide). If there is a deficiency in vitamin B3, the symptoms of pellagra occur.
What is the nicotinamide adenine dinucleotide?
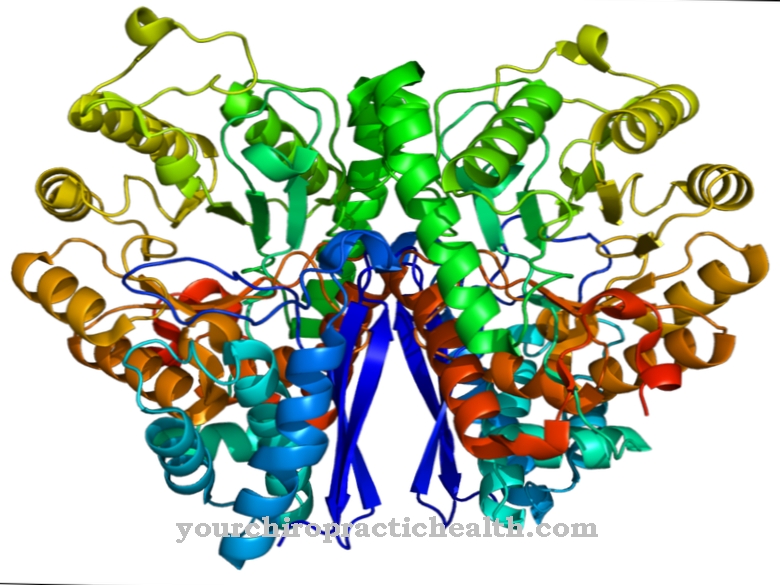
Nicotinamide adenine dinucleotide is a coenzyme that transfers a hydride ion (H-) as part of the energy metabolism. It is present in every cell and especially in the mitochondria. Nicotinamide adenine dinucleotide or NAD is always in the NAD + / NADH balance.
NAD + is the oxidized and NADH the reduced form. In oxidation reactions, NAD + is reduced to NADH by the uptake of a proton (H +) and two electrons (2e-). Formally, this is the transfer of a hydride ion (H-). NADH is very high in energy and transfers its energy to ADP with the formation of ATP. While NAD + is mostly found in the cytosol, NADH is mainly found in the mitochondria. NAD is composed of two nucleotides.
One nucleotide contains the nitrogenous base adenine, while the other nucleotide nicotinic acid amide is glycosidically bound to the sugar. Ribose acts as a sugar. The two nucleotides are connected to one another via the phosphate groups. The ring nitrogen on the nicotinic acid amide residue is positively charged in the oxidized form. This form (NAD +) is lower in energy than the reduced form (NADH) due to the aromatic ring.
Function, effect & tasks
Nicotinamide adenine dinucleotide forms the redox couple NAD + / NADH. The redox potential depends on the ratio of the two components. When the ratio of NAD + / NADH is large, the oxidizing ability is high. The smaller the ratio, the higher the reducing power becomes.
Both oxidation reactions and reduction reactions must take place simultaneously in biological systems. However, this cannot be guaranteed by a single redox couple. That is why the individual reactions with different redox cofactors take place separately. The oxidized form is mainly found in the cytosol, while the reduced form predominates in the mitochondria. Intermediate energy storage takes place again and again within this redox system. With the hydride ion (proton + 2 electrons), NAD + also simultaneously absorbs energy for intermediate storage. The energy comes from the breakdown of energy-rich substrates such as carbohydrates or fatty acids in the respiratory chain.
During the oxidation and release of H-, the energy is transferred to ADP with the formation of energy-rich ATPs. ATP is the most important energy store, which by releasing its energy with regression of ADP either stimulates energy-consuming reactions (build-up of the body's own substances) or mechanical work (muscle work, movement of internal organs) or the body's heat generation. Thanks to its redox potential, the nicotinamide adenine dinucleotide ensures a multitude of redox reactions that enable an orderly production of energy within the respiratory chain. The energy is repeatedly stored temporarily and given off in a targeted manner when required.
Education, Occurrence & Properties
NAD + is biosynthesized from nicotinic acid or nicotinic acid amide (niacin, vitamin B3) as well as from the amino acid tryptophan. Both substances must be absorbed by the body because they are not formed in the metabolism. Tryptophan is an essential amino acid and niacin is a vitamin. If these active ingredients are missing in the diet, deficiency symptoms occur. The daily requirement for vitamin B3 depends on the body's energy expenditure.
The more energy the body needs, the more niacin needs to be supplied. Poultry, fish, dairy products, mushrooms and eggs in particular contain a lot of niacin. Vitamin B3 is also found in coffee, peanuts and legumes. However, deficiency symptoms rarely occur because the amino acid tryptophan can also form NAD. Tryptophan is also found in sufficient quantities in the aforementioned foods. Nicotinate D-ribonucleotide can be synthesized from both starting materials, which is the starting point for the synthesis of NAD +.
Diseases & Disorders
Since the nicotinamide adenine dinucleotide plays a central role in the energy metabolism, its deficiency leads to serious health disorders. In addition to its function as an intermediate energy store, it participates as coenzyme 1 in more than 100 different enzymatic reactions.
In addition to its influence on energy production, it also stimulates the synthesis of the neurotransmitters dopamine, adrenaline or serotonin. It has a stimulating effect in stressful situations, nervousness and tiredness. It also strengthens the immune system, liver functions, nervous system and also acts as an antioxidant. It improves brain functions through the formation of neurotransmitters. Memory, concentration and thinking skills improve. Positive experiences have also been made with Parkinson's disease.
Studies have shown that after administration of NADH there was an improvement in symptoms. A deficiency in NAD is rare today, but it can occur with an extremely one-sided diet.For example, until the beginning of the twentieth century, a mysterious disease called pellagra occurred particularly in Mexico. When the diet switched to corn, a large part of the Mexican population suffered from difficulty concentrating and sleeping, loss of appetite, irritability, skin changes with dermatitis, diarrhea, depression and inflammation of the oral and gastrointestinal mucosa. The reason was the nationwide corn supply.
Both niacin and tryptophan are only found in small amounts in maize. This disrupted the formation of NAD +. After discovering the cause, the diet was changed again. Occasionally, an overdose of vitamin B3 leads to a vasodilating effect, which is also known as flush. You may also experience a drop in blood pressure and dizziness. These symptoms are the expression of an increased energy production by NAD +. However, no toxic effects were observed even at very high doses.





.jpg)

.jpg)


.jpg)