Ranibizumab is a drug from the class of monoclonal antibodies that is used to treat macular degeneration.
What is ranibizumab?

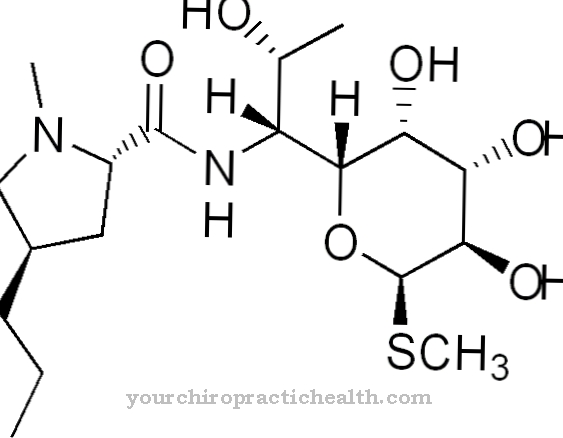
The drug ranibizumab is a monoclonal antibody fragment (Fab). Monoclonal antibodies are antibodies that are produced by a specific cell clone and can only be traced back to a single B lymphocyte. Monoclonal immunologically active proteins play an important role in diagnostics, therapy and research, as they are able to bind a specific number of molecules. In contrast, a physiological immune response always consists of polyclonal antibodies.
Genentech developed and markets the drug ranibizumab. Genentech is a subsidiary of the Swiss pharmaceutical companies Novartis and Hoffman-La Roche. The drug was first approved in 2006 in the USA and Switzerland. In 2007, the EU Commission approved ranibizumab for all EU countries. With the exception of North America, Novartis still has sole distribution rights.
Ranibizumab is produced using recombinant DNA, which is obtained from the E. coli bacterium (Escherichia coli) by genetic engineering. Ranibizumab is a fragment of the monoclonal antibody bevacizumab and prevents the formation of new blood vessels in the eye. Similar active ingredients are also being used more and more frequently in cancer therapy.
Pharmacological effect
The monoclonal antibody fragment ranibizumab has a high affinity for the isoforms of the vascular endothelial growth factor A (VEGF-A) and thus binds to them. VEGF-A appears to be the key molecule in the development of exudative age-related macular degeneration. Because of the binding by ranibizumab, the VEGFR-1 and VEGFR-2 receptors on the surface of the endothelial cells are not activated.
Since ranibizumab has a very small molecule size, it passes through all layers of the retina and thus reaches what is known as choroidal neovascularization (CNV). In macular degeneration, these changes tend to bleed. Ranibizumab prevents the corresponding receptors from being activated and thus inhibits the growth of choroidal neovascularization. As an antibody fragment, ranibizumab also reduces the risk of inflammation in the retina.
Medical application & use
Ranibizumab is used to treat wet age-related macular degeneration (AMD). The drug is also used in the event of a deterioration in visual acuity in the context of diabetic macular edema. In AMD, so-called choroidal neovascularizations form below the retina and bleed quickly. In the final stage, parts of the retina become scarred, so that scars that are under-blooded often develop.
AMD quickly leads to reading blindness. The ability to read decreases, and the perception of contrast and color vision are also limited. The adaptation to changed lighting conditions is difficult, at the same time the sensitivity to glare increases. In more severe cases, central visual field defects can also occur. The diabetic macular edema occurs as part of the metabolic disease diabetes mellitus. If left untreated, this edema can lead to severe visual impairment or even complete loss of vision.
In both diseases, ranibizumab is injected into the vitreous humor of the eye under local anesthesia. The dose is usually 0.05 milliliters. One injection is given monthly for the first three months of treatment. In the following phase, the preparation is only administered in the event of renewed vision loss. In the case of diabetic macular edema, on the other hand, there is a monthly injection until maximum visual acuity is achieved. Since it should only be used under aseptic conditions, only a qualified ophthalmologist may administer the active ingredient.
You can find your medication here
➔ Medicines for visual disturbances and eye complaintsRisks & side effects
Eye problems with floaters, sensation of foreign bodies, pain and bleeding are among the most common side effects. An increase in intraocular pressure with headache or arterial hypertension may also occur during treatment with ranibizumab. Infections of the eye cavity or damage to the retina rarely occur. Antibiotic eye drops may be given to the patient after treatment to prevent infection. In rare cases, cataracts can develop after treatment with ranibizumab.
Despite the rather low rate of side effects, therapy with ranibizumab is more frequently criticized. Studies have compared the two active substances ranibizumab and bevacizumab. It was shown that bevacizumab is just as effective as the much more expensive active ingredient ranibizumab. The use of bevacizumab is also associated with no higher risk or more side effects, so that the use of the more expensive ranibizumab is actually not justified.









.jpg)

.jpg)


.jpg)