Tenofovir (also Tenofovir disoproxil) is used therapeutically for HIV-1 and hepatitis B infections. Tenofovir disoproxil is activated to tenofovir in human cells. On the one hand, it inhibits reverse transcriptase in HIV viruses (or DNA polymerase in hepatitis B viruses) and, on the other hand, is built into the virus DNA as a false component, so that the virus can no longer multiply. It is generally well tolerated, but can lead to kidney failure if there is pre-existing kidney damage.
What is tenofovir?
Tenofovir is an antiviral drug (virostatic) and belongs to the group of nucleoside reverse transcriptase inhibitors (NRTIs) in HIV viruses. The active ingredient can also block DNA polymerase in hepatitis B viruses.
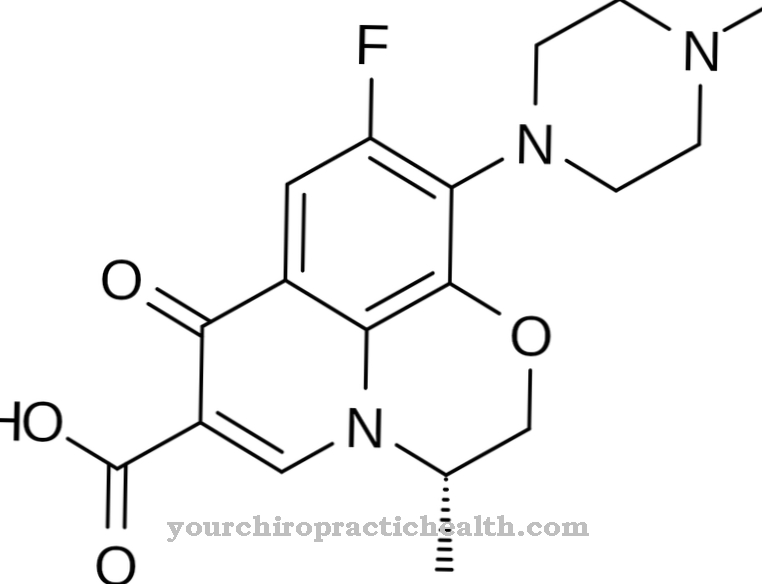
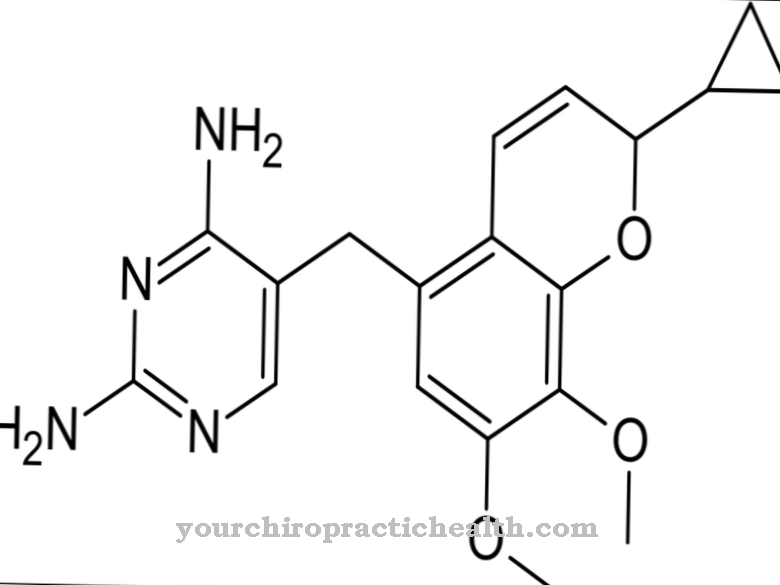
It is a modified adenosine monophosphate analogue and consists of a pentose, a nucleic base and a phosphoric acid residue. Tenofovir disoproxil is the prodrug that is activated to tenofovir by the body's own enzymes.
Pharmacological effect
The drug is taken in tablet form and should be taken with meals. The correct dosage must be discussed with the attending physician and strictly adhered to, as otherwise resistance may develop. There is a low protein binding and the plasma half-life is 12 to 18 hours. It is mainly excreted via the kidneys.
The active ingredient tenofovir disoproxil is absorbed unchanged into the human cell and phosphorylated and activated by special enzymes, the kinases, in nucleotide triphosphates. Tenofovir has a dual mechanism of action. The activated derivatives inhibit, on the one hand, the viral reverse transcriptase in the HI virus and the DNA polymerase in hepatitis B. As a result, the DNA synthesis is now aborted due to the missing 3 'hydroxyl group on the activated tenofovir. This prevents the virus from multiplying.
However, there are also DNA polymerases in the human body, especially in the mitochondria. These can also be inhibited by the drug, with corresponding side effects.
Medical application & use
Tenofovir is used to treat HIV-1 and hepatitis B infections. The drug was initially approved for HIV therapy in Europe in 2002 and has been indicated for the treatment of chronic hepatitis B since 2008. Tenofovir is used in particular in patients with hepatitis B and active virus replication and elevated liver enzymes.
Tenofovir is always used in combination with other drugs in the treatment of HIV. The therapy can be used in adults as well as in adolescents aged 12 to 18 years.
Tenofovir can reduce the transmission of the virus to the unborn child in pregnant women with chronic hepatitis B infection. Under study conditions, the drug was given in the last trimester of pregnancy and continued up to 4 weeks after the birth. A significant increase in malformations in the unborn could not be observed until then.
It should be noted that treatment with tenofovir does not cure HIV-1 or hepatitis B, so the patient can still transmit viruses to other people even during treatment. Appropriate protective measures are therefore essential to avoid infection.
Risks & side effects
In general, tenofovir is very well tolerated. Common side effects are nausea, diarrhea, tiredness, dizziness and headache. However, caution is advised in patients with pre-existing renal impairment. The agent has a nephrotoxic effect and in rare cases can lead to kidney failure. Tenofovir should also not be taken with other drugs that can cause additional damage to the kidneys. Tenofovir is contraindicated in dialysis patients.
Inhibiting human mitochondrial DNA polymerase can cause some rare but important long-term side effects. Infants who have been exposed to nucleoside therapy in the womb, in particular, may have an increased risk of side effects. Lactic acidosis can occur due to an excess of lactic acid in the blood. This manifests itself in deep and fast breathing, drowsiness as well as nausea, vomiting and stomach pain. This should be treated immediately by a doctor as this side effect can be fatal.
Furthermore, inflammation of the pancreas (pancreatitis) can occur, which is particularly noticeable through pain in the upper abdomen. In rare cases muscle and joint weakness, damage to the nerve tracts (polyneuropathy) and lipodystrophy (body fat redistribution) can occur. If there is a known allergic reaction to tenofovir itself or to other components of the drug, it must not be taken.
Pregnancy poses a particular challenge and requires an individual, medicinal approach. Breastfeeding during therapy is not permitted as it is not yet known whether the drug is excreted in breast milk.