After a bleeding injury, the Platelet aggregation recovery as an important step in wound care. It ensures that platelets accumulate within a few minutes, stick the damaged area together and in this way let the blood flow ebb.
What is platelet aggregation?

Platelet aggregation is the name given to an essential part of the process during blood clotting. In the first phase of blood coagulation (primary hemostasis), the platelets (Greek: thrombos, clumps) ensure primary wound closure by clumping and accumulating (Latin: aggregare, to accumulate).
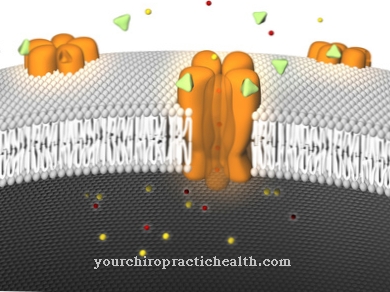
The process is accompanied by a narrowing of the affected blood vessels and those of the surrounding area. The blood platelets (thrombocytes) change their appearance and the properties on the cell surface as they clump together. The change in shape of the platelet exposes receptors on the surface that are now active. The activated platelet can adhere to the vessel wall through them.
In addition, other processes take place that support hemostasis. Any factors released on the damaged vessel wall direct the platelets to this point. In addition, substances are released that prevent inflammation and initiate the next steps in blood clotting. They ensure permanent wound closure and ultimately healing.
Function & task
Platelet aggregation prevents major blood loss after an injury. This process is part of the blood clotting system. This system works as a complex and finely tuned interplay of different cells (platelets), coagulation factors and several messenger substances. It works like a chain reaction.
Coagulation factors are predominantly proteins that are activated under certain conditions and in turn initiate or accelerate reactions within the coagulation process. In medical use, the coagulation factors have been given Roman numbers (from 1 to 13).
The platelets initiate the coagulation reaction if the surface is damaged. The process behind it takes place in three phases. The adhesion (Latin: to stick) as well as the aggregation of platelets and the formation of a plug that closes the wound. The cell walls of the injured vessels or tissue release a coagulation-active factor, the so-called Von Willebrand factor. This is a protein molecule that is synthesized by the cells in the inner vessel wall (endothelial cells) and the precursor cells of the blood platelets. It is stored in the platelet and released when activated. This factor is responsible for the adhesion of the blood platelets (sticking to the vessel wall) so that they thinly cover the wound.
At the same time, platelet aggregation is initiated in this way. This happens because after the activation of the blood platelets, genes are also activated that set the synthesis of a receptor necessary for aggregation in motion. With the help of the structural protein collagen, thrombin, an important enzyme in blood clotting, the nucleotide adenosine diphosphate (ADP), hormones such as adrenaline and other endogenous substances, the platelets change their shape. In the process, further components are released and the affected area is prepared for the next steps in blood clotting.
A cascade of different factors is activated. While the platelet aggregation is initially reversible, a level is finally reached at which the platelets network with the participation of a special protein (fibrinogen, factor I) and an irreversible thrombus (blood clotting plug) forms.
Illnesses & ailments
Disturbances in platelet aggregation can manifest themselves as an increased or decreased reaction. They can occur in patients with a hereditary predisposition or after taking certain medications. The congenital diseases are rare and affect the platelet aggregation itself or various processes accompanying the process.
Those affected become conspicuous by spontaneously occurring mucosal and nasal bleeding as well as by their tendency to hematomas (bruises). Female patients suffer from heavy menstrual bleeding and birth bleeding.
One of these congenital diseases was named after the Swiss pediatrician E. Glanzmann: Glanzmann-Naegeli's disease (also Glanzmann's thrombasthenia). It is inherited as an autosomal recessive trait. Affected is a receptor in the platelet membrane that is not made available in sufficient quantities due to a genetic change (mutation). Patients with this defect are at great risk from taking platelet aggregation inhibitors such as Aspirin®.
In the Willebrand-Jürgens syndrome, the factor that is important for the adhesion and aggregation of the platelets is not present in sufficient quantities or with qualitative limitations. This means that it is not fully functional and the platelet adhesion as a preparatory step in aggregation is impaired.
Two French hematologists are the namesake of another inherited, very rare platelet disorder: the Bernard-Soulier syndrome. Primarily the adhesion of the blood platelets is affected. It is reduced and in this way also reduces platelet aggregation.
Patients with storage pool disease show impaired secretion performance after activation of the platelets. This is due to the missing granules. These are cellular deposits (vesicles) from which the various factors are released during the activation of the blood platelets. The gray platelet syndrome (gray platelet syndrome) is a special form.
Acquired or medicated disorders of platelet aggregation are also diagnosed more frequently. So-called exhausted blood platelets, which are no longer able to aggregate, can occur in dialysis patients, through heart-lung machines, in severe kidney diseases or after burns. The situation in these cases is similar to that of storage pool disease.
Increased platelet aggregation is found in coronary heart disease, after strokes, in vascular diseases and acute thrombosis. Drugs that inhibit platelet function are often used for thrombosis prophylaxis. Acetylsalicylic acid (e.g. in aspirin) is one of them. There are also some chemotherapy drugs that reduce platelet aggregation.





.jpg)

.jpg)


.jpg)