Acetates are chemical substances that are often used in the text industry and pharmaceuticals. The acetic acid salts occur in various forms, including as metabolic products in the human body. Together with esters, derivatives of carboxylic acid, free acetic acid is produced as a component of blood and tissue.
What are acetates?
Acetate or also Ethanoates denote the esters and salts of acetic acid. Other components include substances such as aryl, arylalkyl and alkyl, which form organochemical residues. These residues from the original substances result in the acetates with their associated structure in relation to the chemical composition.
The intestinal bacterium Bacteroides thetaiomicron, for example, is able to convert dietary fiber into the fatty acid acetate. About ten percent of the daily calorie consumption comes from such fatty acids produced by microbes. Acetates exist in different forms such as sodium acetate, calcium acetate, lead acetate or ammonium acetate. The individual acetate types have slight differences in their chemical system, but have the same properties and similar compositions. Acetates are found in almost every living matter.
In a biological organism, the acetate acetyl-coenzyme A acts as an intermediate product and is involved in a large number of metabolic reactions. Salts and esters of acetic acid are widespread in nature, but they are extremely rare in natural minerals. However, some minerals such as calcanite and hoganite are based on acetate.
Function, effect & tasks
Vitamin E acetate (tocopheryl acetate) plays an essential role in maintaining body functions. In the case of certain illnesses or deficiency symptoms, vitamin E preparations are therefore used in the form of synthetically produced derivatives because they are particularly stable and long-lasting.
The fluids produced with a very high level of purity are often used in the manufacture of medicines. Vitamin E acetates are also used as additives in the cosmetics industry and in animal feed production. Tocopheryl acetate as capsules or dragees should only be taken under medical supervision. Stable vitamin E acetates can also be found in creams for dry skin or in sunscreens. Sodium acetate is used as a food additive and may be added up to the specified maximum amount. However, this acetate is not approved for untreated foods, as these products may not contain any additives according to legal guidelines. Sodium acetates are found in dairy products, canned vegetables or mayonnaise.
The colorless salt, which smells slightly of vinegar, also serves as a buffer to give melting salts or technical materials the right degree of acidity. Sodium acetates are therefore able to neutralize the sour taste in a food. In addition, this acetate can curb the growth of some types of bacteria. However, the formation of mold cannot be prevented by using sodium acetate. The salts are often used as additives in combination with various preservatives. In the body, acetates are further digested into acetic acid. The human organism needs activated acetic acid to generate energy so that the cells continuously generate enough energy to withstand environmental influences.
Education, occurrence, properties & optimal values
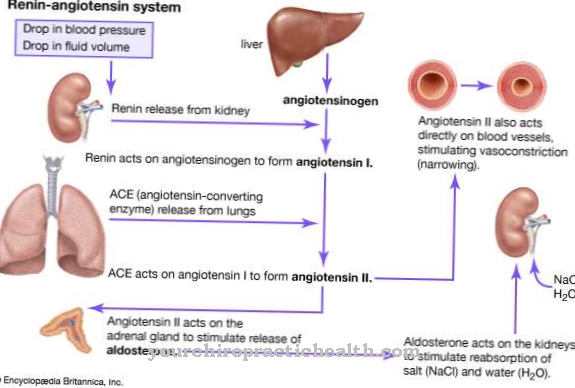
The citric acid cycle is a system of biochemical reactions that plays a central role in numerous metabolic processes that build up and break down. With regard to the regulation of the metabolism, this means that high concentrations of the original products have an activating effect on the body. This also includes acetic acid. The acetate or the salts and esters of acetic acid have a significant influence on metabolic processes. More precisely, it is the activated acetic acid acetyl-CoA, where the abbreviation CoA stands for coenzyme A.
This factor is involved in a large number of enzyme reactions. If acetic acid is converted into acetyl-CoA, carboxylic acid can develop its full biochemical usefulness. Acetyl-CoA not only functions as an important control point in metabolic processes, but is a basic element for the connection of sex hormones, adrenal cortical hormones and tissue hormones such as histamine and serotonin. Vinegar is often used as a natural remedy to stimulate digestion and metabolism. The digestive process speeds up because vinegar stimulates the secretion of salivary glands. This promotes the uptake of nutrients and promotes the elimination of degradation products.
Diseases & Disorders
Like sodium acetate, calcium acetate is also used as a preservative and acid-regulating food additive. The calcium salt of acetic acid is also used as an extraction solvent for soil samples and as fuel paste in connection with denatured alcohol.
In medicine, calcium acetate is used in the treatment of chronic kidney failure or in the event of an increased phosphate level. Calcium acetate solutions are also contained in heat packs. As the metal platelets deform in the cushions, a crystallization nucleus forms. The complete acetate solution crystallizes within a few seconds, but can store and release heat over a longer period of time. The duration of the heat release depends on the size and volume of the heat cushion and the ambient temperature. The drug calcium acetate is a mineral preparation that binds phosphates contained in food in order to reduce phosphate absorption in the organism. The medicine is given to patients who are on dialysis because they have severe kidney disease.
The drug calcium acetate must not be taken if the calcium concentration in the blood is too high. Hypercalcemia often occurs when the parathyroid glands are overactive. Overdosing with vitamin D, certain types of blood cancer, bone metastases, calcium-containing kidney stones and various lung diseases increase the calcium concentration, which is why the intake of calcium acetate poses a health risk. In the textile industry, acetate fibers produced using the dry spinning process are used to manufacture raincoats, umbrellas, blouses, shirts, women's underwear and ties. The man-made fibers dry very quickly, are extremely flexible and insensitive to fungal attack. In contrast to polyester, acetate absorbs a lot of moisture and releases it back into the outside air.



.jpg)




















