Adefovir is a medicine used to treat hepatitis B. Long-term use prevents the hepatitis B viruses from multiplying.
What is adefovir?

Adefovir, also known as adefovirum, belongs to the class of antivirals. These are medicines that stop viruses from multiplying.
Adefovirum was approved in the European Union in 2003. It is prescribed to adults for the treatment of chronic hepatitis B. Usually, the drug is only used if there is also a liver disease. This could be a disorder of the serum values or an inflammation of the liver.
The drug is marketed in Germany under the name Hepsera. The half-life of the active ingredient is seven hours, after which it is broken down by the kidneys. Adefovir is only slightly bound by proteins in the blood.
Pharmacological effect
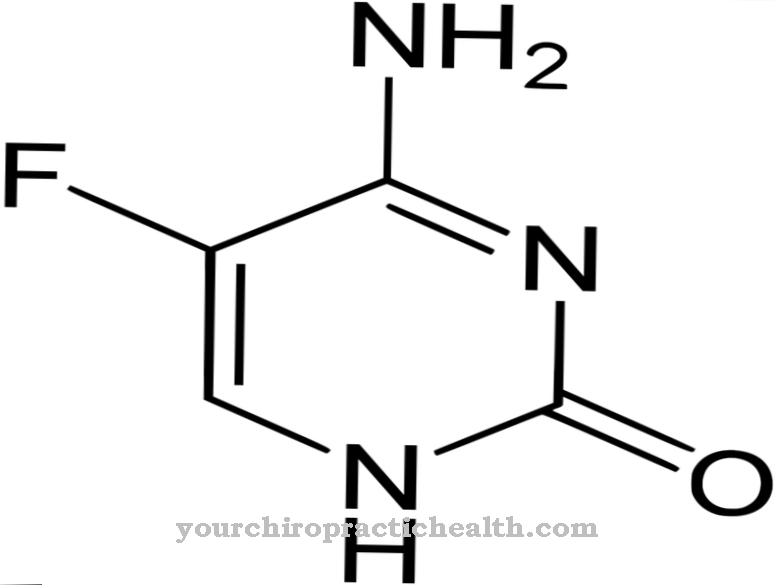
In medical circles, adefovir is classified as a prodrug. This is an initially inactive ingredient that only takes effect after ingestion. After ingestion, adefovir is converted to adenosine monophosphate in a transition state.
The phosphate forms a related structure, but it is better absorbed by the infected cells. There it is eventually converted to adefovir diphosphate and takes on its active form. Inside the cell, the adefovir diphosphate collides with the naturally occurring substrate deoxyadenosine triphosphate. Since both compounds are very similar, nucleic acid synthesis is impeded. As a consequence, the infected cell is prevented from dividing.
Overall, the rate at which the viruses multiply is reduced. Colloquially, this procedure is also known as suicide inhibition. Since this method can also be used to stop human DNA polymerase, only low concentrations of the active ingredient may be taken. Incidentally, a continuous increase in resistance can be observed during treatment.
This is due to a mutation of a polymerase gene. In the long term, the clinically observed resistances can minimize the success of the treatment. Therefore, a reduction in the viral load is only possible for a short time. Usually this is enough to prevent further liver damage.
Medical application & use
Adefovir is a prescription medicine. It is only used to treat chronic hepatitis B disease. The drug Hepsera, which is sold in Germany, contains the active ingredient in the form of tablets. These are taken orally as directed by the doctor. A bioavailability of around 60 percent can be expected. This means that the proportion of the active ingredient makes up 60 percent of the total amount.
However, the drug is associated with low protein binding. So the circulation is less than four percent of the ingested amount available. After a few hours, adefovir is cleared again. This is done through the kidneys through filtration and secretion. A half-life of seven hours can be expected here. According to this, half of the amount of active ingredient ingested leaves the body after every seven hours.
However, it should be noted that the drug is only prescribed in combination with an upcoming or ongoing liver disease. In addition, active virus replication must be demonstrated. This means that the progression of hepatitis B disease should be checked during initial or subsequent treatment. Depending on the medical history, there may be exceptions.
Risks & side effects
Treatment of adefovir has a number of side effects. One of the main side effects is nephrotoxin. It is known colloquially as kidney poison. The name goes back to the toxic effects of the drug, especially against kidney cells.
Therefore, kidney function must be checked at regular intervals. If a restriction is found, the doctor can adjust the recommended dose. In addition, gastrointestinal complaints can occur. These are disorders of the digestive system. Long-term use can lead to headache and neck pain.
These decrease again after the end of treatment. Adefovir is also unsuitable for use by minors and pregnant patients. A risk-benefit assessment can be made under certain circumstances. Often the consequences of the therapy outweigh the success of the treatment. It is still unknown whether the agent occurs in breast milk. As a precaution, breastfeeding should be avoided during the entire duration of treatment.