Aspartic acid is a non-essential amino acid that is sufficiently supplied with the diet. It is part of most proteins. In addition to glutamate, aspartic acid acts as a neurotransmitter.
What is aspartic acid?
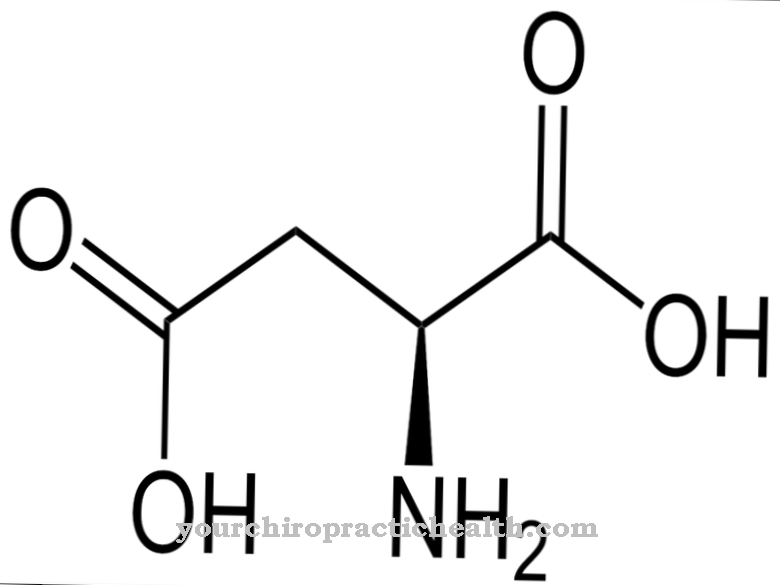
Aspartic acid is a non-essential amino acid that is sufficiently present in all protein-containing foods. It contains two acid groups and is therefore an acidic amino acid.
Their biosynthesis takes place in the body very simply from oxalic acid through transamination. It occurs in two optically active forms, with D-aspartic acid having no biological significance. Only L-aspartic acid is a proteinogenic amino acid. Whenever aspartic acid is mentioned in the following, the L-form is always meant. In biochemistry, it is often referred to as L-aspartate because it is usually deprotonated in the body. In the urea cycle, aspartate serves as an amino group donor. Aspartic acid is also produced industrially by the addition of ammonia to the double bond of fumaric acid.
It is of great importance as a raw material for the production of the sweetener aspartame. Aspartame is a dipeptide made from the amino acids aspartic acid and phenylalanine. It is also used for parenteral nutrition in infusion solutions or as a salt former. Their technical use as polyaspartic acid esters in modern paint systems is also of interest.
Function, effect & tasks
The most important function of aspartic acid is its participation in the building of proteins. It is one of the 20 proteinogenic amino acids. In addition to glutamate, L-aspartate acts as a neurotransmitter in over half of all synapses in the central nervous system of vertebrates.
The exact mode of action of aspartic acid has not yet been precisely researched. It is said to be active in the climbing fibers of the cerebellum and in the moss fibers of the ammonia formation. Overall, however, it is said to have a weaker effect than glutamate. Aspartic acid works by stimulating the NMDA receptors. It is also a starting material for the formation of nucleic bases. It is available for the synthesis of the pyrimidine bases. In the urea cycle, aspartic acid is converted into argininosuccinate with the aid of the enzyme argininosuccinate synthetase. Argininosuccinate is a metabolite of the urea cycle.
It is a non-proteinogenic amino acid which is broken down by the enzyme argininosuccinate lyase into the proteinogenic amino acids arginine and fumarate. As part of the urea cycle, L-arginine releases ammonia. The ammonia released by L-arginine is converted into urea, which is excreted by the kidneys. Fumarate converts back to oxaloacetate (oxalic acid). The oxalic acid is transaminated again to aspartic acid with the help of an alpha amino acid. Glutamic acid is usually available for this, which is then converted into ketoglutarate.
Education, occurrence, properties & optimal values
Aspartic acid is widely used. It is difficult to imagine malnutrition that leads to a deficiency in aspartic acid. L-aspartate is found in all protein-containing foods. Particularly high concentrations are found in vegetable asparagus.
The asparagus, with its Latin name Asparagus officinalis, gives its name to the amino acids asparagine and aspartic acid. Very high levels of L-aspartate are also found in legume seedlings, soy protein, dried egg white, cod, peanut flour, dried spirulina, tofu and also sunflower seed flour. However, it does not need to be supplied through food.
Aspartic acid is one of the amino acids that can also be sufficiently synthesized in the metabolism. Even if L-aspartate were not taken in with the diet, there would not be a deficiency, because it is one of the most simply structured and easily synthesized amino acids.
Diseases & Disorders
The main health effect of aspartic acid is to convert ammonia into urea via the urea cycle and to remove it from the body. The additional intake of L-aspartate is said to help improve ammonia detoxification.
Studies have allegedly found that aspartate has positive effects on states of exhaustion, tiredness and low exercise capacity. However, the research results are not so clear that the effect can be conclusively assessed. However, indications have been found that a low concentration of aspartic acid in the organism is related to stressful situations and states of exhaustion. Together with lysine, aspartic acid can also be used for heavy metal removal through complex formation with heavy metals.
There are contradicting statements about possible negative side effects when taking excessive doses of l-aspartate. According to some sources, there are no side effects, while other reports speak of severe nerve damage. Effects on nerve function are suspected because aspartic acid acts as a neurotransmitter alongside glutamate. However, no clear statements have been made on this so far. The sweetener aspartame has caused much discussion. Aspartame is a dipeptide made from phenylalanine and aspartic acid. Studies have been conducted and the results are controversial.
After consuming sweetened foods and drinks with aspartame, isolated cases of migraines, other headaches, mood disorders, depressive moods and many more have been described. However, a connection with the sweetener could not be proven and in some cases even excluded. However, there is a clear contraindication to aspartame for people with phenylketonuria. In phenylketonuria, the amino acid phenylalanine leads to severe metabolic disorders.
With this disease, a special diet low in phenylalanine must be followed. The frequency of this disease is about 1 in 8000. Therefore, aspartame is labeled as containing phenylalanine. However, this contraindication has nothing to do with the aspartic acid contained in aspartame.Overall, it can be said that there are quite contradicting statements for aspartic acid with regard to health effects, which do not allow a final assessment.