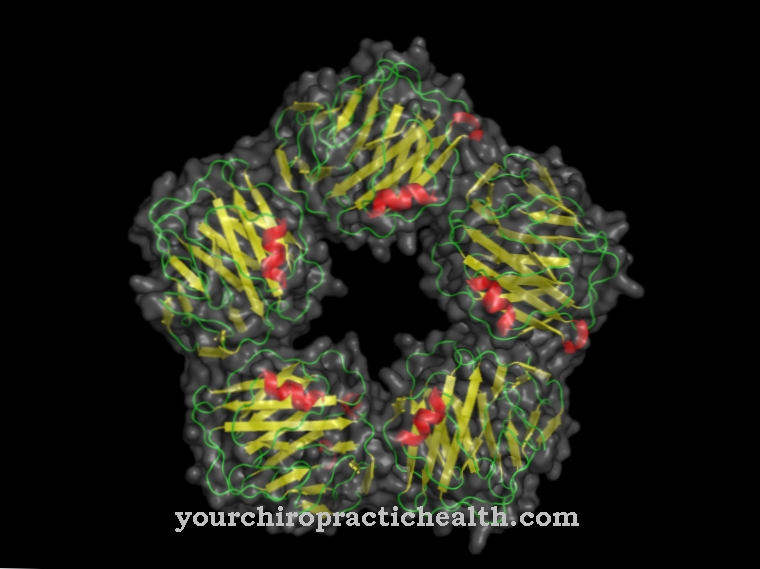
Calcineurin (CaN) is a protein phosphatase that plays an important role in activating the T cells of the immune system, but is also active in other calcium-mediated signaling pathways throughout the body. By dephosphorylating the NF-AT protein, this enzyme initiates a series of gene transcriptions that are mainly responsible for the characteristic work of the T lymphocytes. Thanks to this key position, calcineurin is the starting point for several therapeutic methods of immunosuppression.
What is calcineurin?
The enzyme is made up of two subunits: Calcineurin A (approx. 60 kDa) takes care of the catalytic function and has a calmodulin binding site, while calcineurin B (approx. 19 kDa) is regulatory active and has two calcium ion binding sites.
In its ground state, CaN is inactive because part of the protein blocks the active center - this is called autoinhibition. The binding of calcium-activated calmodulin and calcium ions is required for complete activation. As a phosphatase, calcineurin is assigned the EC number 3.1.3.16, which comprises those enzymes that catalyze the hydrolytic dephosphorylation of serine and threonine residues of other proteins.
Function, effect & tasks
The substrate binding site of the enzyme is selective especially for NF-ATc (nuclear factor of activated T-cells, cytosolic). This transcription factor is found in the cell plasma of lymphocytes. In the ground state, NF-Atc is phosphorylated and therefore inactive.
The role of calcineurin in the immune response begins with the uptake of an antigen - e.g. of a virus, a bacterium or components of degenerated cells - by a cell of the immune system (monocytes, macrophages, dendritic cells and B cells). This substance is then processed and presented on the surface of the cell.
When antigen-presenting cells come into contact with the T-cell receptor of the T-cells, a signal cascade is set in motion. These extracellular stimuli increase the calcium concentration in the cell. Calcium ions bind to CaN B, which by changing the structure of the protein dissolves the autoinhibitory domain of CaN A and mediates the calmodulin binding to CaN A. This makes calcineurin fully catalytically active and dephosphorylates the serine-rich region (SRR) in the amino terminus of NF-ATc. This results in a conformational change of NF-ATc, as a result of which the transcription factor is transported into the cell nucleus. There it triggers the transcription of several genes that are responsible, among other things, for the production of interleukins such as IL-2.
IL-2 also ensures the activation of T helper cells and the synthesis of cytokines, thus directing the work of cytotoxic T cells. While the helper cells control other lymphocytes in the immune response - e.g. by maturation of B cells to plasma cells or memory cells and activation of phagocytes - cytotoxic T cells are responsible for the destruction of infected or degenerated cells in the body. Since this path cannot be followed without calcineurin, the enzyme plays a key role in the immune response.
Further target proteins of the enzyme are the cAMP response element binding protein (CREB) with influence e.g. on the nervous system and the internal clock and myocyte enhancer factor 2 (MEF2), which is partly responsible for cell differentiation in embryonic development and plays a role in the stress response of some tissues in adults.
Education, occurrence, properties & optimal values
There are different isoforms of the two subunits (CaN A: 3 isoforms, CaN B: 2 isoforms), some of which are expressed differently depending on the body region. In particular, CaN A γ stands out, which occurs exclusively in the testes and is involved in seed maturation there. Despite the important role it plays in the immune system and nerves, one can assume that calcineurin can be found in almost all tissues. The regulation takes place less via an increase or decrease of the synthesis but via the calcineurin inhibitor CAIN. This prevents e.g. the dephosphorylation of NF-AT.
The negative feedback regulation by RCAN1 ensures that no excessively high cytosolic concentration of CaN occurs. Activated (dephosphorylated) NF-AT binds to the gene promoter of RCAN1 in the cell nucleus and thereby triggers transcription. The resulting RCAN1 binds to CaN and inhibits its activity.
Diseases & Disorders
Calcineurin is the target of calcineurin inhibitors such as Cyclosporine, pimecrolimus and tacrolimus. By inhibiting the phosphatase action of CaN, an immunosuppression is caused, which e.g. after organ transplants to reduce the likelihood of rejection or in autoimmune diseases to combat inflammatory processes.
Thus, CaN inhibitors are also used for the treatment of diseases from the rheumatoid group. Other approaches that are currently being explored are the fight against tuberculosis, schizophrenia and diabetes. The exclusive presence of CaN A γ in the testes implies a possible role in the development of contraceptives. In cases of cardiac hypertrophy in which the CaN-NA-FT signal pathway is involved, the development of hypertrophy could be prevented by administration of CaN inhibitors.
People with Down syndrome have three 21 chromosomes instead of the usual two, which encode a calcineurin-inhibiting protein. This inhibitor prevents calcineurin from interacting with cells of blood vessels and triggering proliferation processes in these. This fact is particularly important in the case of tumors, as these, among other things, ensure their blood supply via calcineurin. Intervening at this point can effectively prevent the progression of cancer. So you can find e.g. a significantly lower incidence of tumors in people with Down's syndrome and hopes that targeted inhibition of this process will provide advantages in the fight against cancer in the future.
Recently, there is also increasing evidence that age-related dysregulation of calcineurin could also play a role in the development of neuronal diseases such as Alzheimer's. Research into the signaling pathways in which the enzyme is involved reveals more and more white spots on the biochemical map. At the same time, it opens up the hope that with the help of this key protein we will be able to better understand and treat a large number of different diseases in the future.

.jpg)