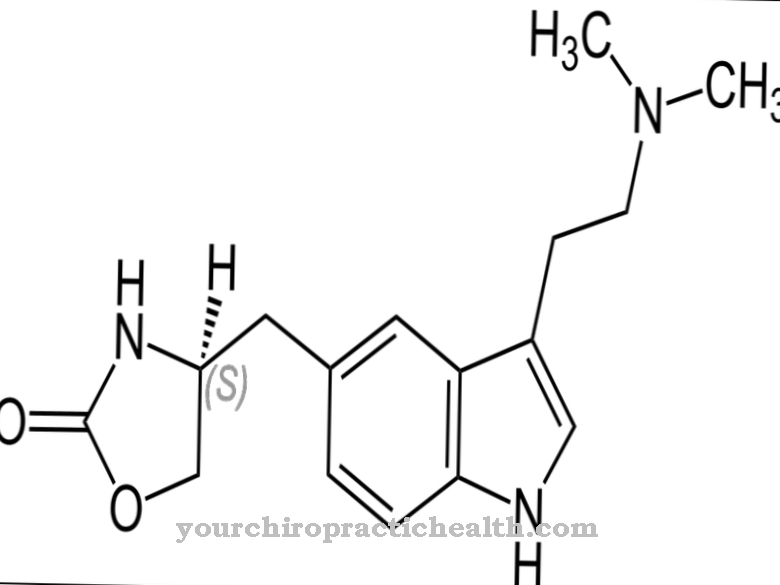
As Capecitabine is called a cancer drug. It belongs to the group of cytostatics.
What is capecitabine?
Capecitabine is a cytostatic drug that is used to treat cancer. The active ingredient is a prodrug (precursor) of 5-fluorouracil (5-FU). Within the tumor, it is converted into an active substance.
Capecitabine is administered orally and is suitable for the treatment of metastatic or advanced breast cancer, metastatic colon cancer and the palliative treatment of gastric cancer.
Capecitabine has been approved in the USA and Switzerland since 1998. In 2001, the drug was also approved in Germany. The cytostatic is sold under the trade name Xeloda®. The drug has also been available in generic form since 2013.
Pharmacological effect
Capecitabine belongs to the group of antagonists of pyrimidine and purine bases. As a precursor to 5-fluorouracil, it is of great importance for the treatment of tumor cells. The effect of the cytostatic can be compared with that of 5-FU.
The enzyme thymidine phosphorylase converts capecitabine into 5-fluorouracil. This occurs in high concentrations within the tumor tissue. Since the effect of action is directly directed towards the tumor cells, capecitabine can be better tolerated by cancer patients. As a result, there are fewer side effects that require treatment.
Capecitabine works by inhibiting the division of the degenerated cancer cells. Due to the inability of the cells to divide, the growth of the tumor is ideally brought to a standstill. The active ingredient is rapidly absorbed in the gastrointestinal tract. This reaches its maximum plasma concentration after about 90 minutes. The plasma half-life of capecitabine is approximately 40 minutes.
In the liver, capecitabine is hydrolyzed to 5-deoxy-5-fluorouridine. In the further course, the conversion into 5-fluorouracil finally takes place. Later, around 95 percent of the 5-FU is eliminated from the body through the kidneys. The rest of the elimination takes place in the stool.
Medical application & use
Capecitabine is administered as a single active ingredient against colon cancer.However, a combination therapy with other cytostatics can also be carried out. Capecitabine treatment is also considered sensible if the colon cancer has already led to the formation of metastases (daughter tumors).
Another area of application for capecitabine is the initial treatment of advanced gastric cancer. As part of the treatment, it is combined with active ingredients that contain platinum, such as cisplatin.
The indications for the cytostatic also include locally advanced breast cancer or metastatic breast cancer, whereby capecitabine is usually combined with the taxane docetaxel. Such treatment only takes place if other chemotherapeutic measures were unsuccessful beforehand. Capecitabine can be used as a single active ingredient if taxane therapy was ineffective or treatment with anthracycline appears unsuitable.
Capecitabine is taken in the form of film-coated tablets. The patient takes this in the morning and in the evening half an hour after a meal. Depending on how high the doctor sets the dose, it may be necessary to swallow 3 to 7 tablets. If severe side effects occur, the dose must be reduced or the treatment interrupted.
Risks & side effects
Compared to 5-FU, the side effects of capecitabine are lower. This applies primarily to inflammation of the lining of the mouth (stomatitis), nausea, vomiting and hair loss.
Nevertheless, various undesirable side effects are also possible with this cytostatic agent. These include abdominal pain, diarrhea, a reduction in lymphocytes, inflammation of the skin, an increase in the bile pigment bilirubin and fatigue. It is not uncommon for a hand-foot syndrome to appear, which manifests itself in symptoms such as abnormal sensations, tingling, numbness and severe pain in the hands and feet. Sometimes blisters or ulcers will also develop on them. Cold hand and foot baths and the application of creams that contain uridine are helpful antidotes.
Other possible side effects include indigestion, flatulence, dry mouth, itching, dry skin, headache and body aches, feelings of weakness, taste disturbances, dizziness and the formation of edema (water retention).
Difficulty breathing, depression, high blood sugar, fever, back pain, nosebleeds or weight loss can also occur. In the worst case, a heart attack is even possible. If severe skin reactions occur during capecitabine therapy, it must be stopped immediately in consultation with the doctor.
If the patient suffers from hypersensitivity to capecitabine or 5-FU, no treatment with the cancer drug should be given. This is also the case if there is a deficiency in the enzyme dihydropyrimidine dehydrogenase. Further contraindications are pronounced kidney and liver dysfunction and a reduced number of blood cells such as platelets and leukocytes. In the case of severe heart disease such as a weak heart muscle or cardiac arrhythmia, diabetes mellitus or diseases of the nervous system, the doctor must carefully weigh up the risks and benefits.
Capecitabine must never be used during pregnancy and breastfeeding. The child is at risk of serious harm. In principle, the active ingredient is not suitable for the treatment of children and adolescents under 18 years of age.
Interactions with other drugs must also be taken into account. For example, concomitant therapy with capecitabine and herpes medication of the brivudine type must be avoided. This also applies to treatment with phenytoin, an anti-epilepsy drug. Its use may result in phenytoin poisoning.
If anti-coagulants such as phenprocoumon or warfarin are taken at the same time, this changes the properties of the blood. As a result, complications such as nosebleeds, blood in the urine or stool and vomiting of blood are conceivable.







.jpg)

.jpg)


.jpg)