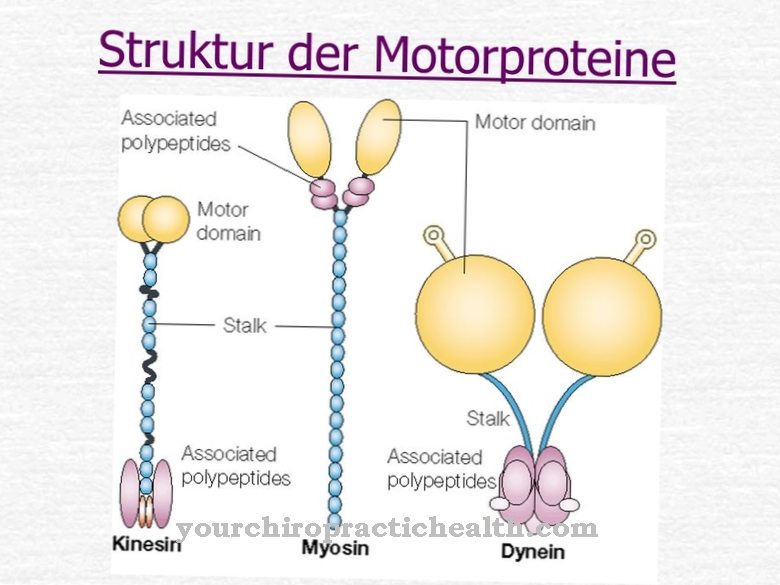
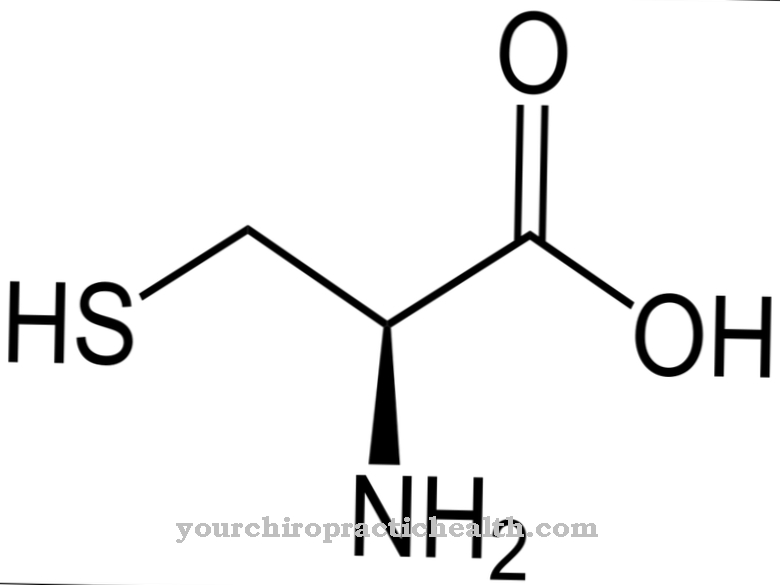
At Hydrolase is a group of enzymes that hydrolytically break down substrates. Some hydrolases contribute to the normal functioning of the human body, for example the starch-splitting amylase. Other hydrolases are involved in the development of diseases and, like urease, are produced in bacteria.
What is the hydrolase?
Hydrolases are enzymes that use water to break down substrates. The substrate docks to the active center of an enzyme, where interactions between the two units cause the substrate to break down into two parts. At the same time, a water molecule (H2O) is divided into a single hydrogen atom (H) and an OH group. One part of the substrate attaches to the single hydrogen atom, while the OH group attaches itself to the other part of the substrate. The hydrolase product accordingly consists of two new compounds.
Hydrolases work with different substrates; these include esters, ether peptides, glycosides, acid hydrides and C-C bonds. The hydrolytic cleavage by hydrolases is reversible, i.e. reversible. In the EC classification, they represent group 3, which comprises several subgroups. The subgroups include, for example, lipase, which breaks down fat, and lactase, which breaks down milk sugar (lactose). A lack of lactase leads to intolerance of milk sugar, which can be reflected in gastrointestinal complaints when consuming milk.
Function, effect & tasks
Hydrolases are numerous in the human body. Amylase is also one of the hydrolases. Amylase is found in saliva and is responsible for breaking down starch and other polysaccharides. Polysaccharides are multiple sugars made up of chains of carbohydrates.
Amylase hydrolytically splits these chains and breaks them down into smaller units. This creates the sweet taste that people can taste when chewing bread and other starchy foods. The processing of polysaccharides by amylase is the first stage of biochemical digestion - after the teeth have mechanically crushed the food while chewing.
The kynureninase occurs in all tissue types and splits alanine. Both the synthesis of nicotinic acid and the breakdown of tryptophan require this step. Tryptophan is an essential amino acid that is involved in the synthesis of serotonin. Serotonin is an important neurotransmitter (messenger substance). The breakdown of tryptophan is also an intermediate step in the synthesis of other substances, for example nicotinamide adenine dinucleotide (NAD).
NAD is a coenzyme that participates in numerous biological functions. For example, it supports the work of dehydrogenases and is part of the respiratory chain. Kynureninase not only contributes to the breakdown of tryptophan, but also to the synthesis of nicotinic acid. Nicotinic acid or niacin is a vitamin that is part of the B complex.
Education, occurrence, properties & optimal values
The human body forms hydrolases where they are used. For example, the amylase in saliva is produced in the salivary gland, while the pancreas produces the pancreatic amylase. Like all enzymes, hydrolases can only work under certain conditions. Above all, the pH of the environment and the temperature are of great importance to them.
For example, amylase can only exist at pH 3.5-9. If the milieu deviates from this range, the enzyme denatures. The gastric acid has a pH value of 1–1.5 on an empty stomach and is therefore too acidic for amylase. The stomach acid denatures the protein structure by breaking molecular bonds. The enzyme loses its shape and becomes inactive. This is why the pancreas must also synthesize amylase and add it to the pulp at a later stage of digestion.
The temperature optimum for amylase is 45 ° C; At this temperature, the amylase works fastest, i.e. it converts the largest amount of substrate. Amylase can also work outside of this optimum - but the metabolic rate is somewhat lower. Temperatures that are too high also denature the enzyme and either make it unusable or break down the protein into its individual amino acids.
Diseases & Disorders
Some hydrolases can help diagnose diseases. For example, doctors can use amylase levels in the ovaries and lungs to diagnose certain forms of cancer. The amalysis concentration is noticeable in cancer in these organs and can therefore provide an indication of the presence or spread of neoplasms.
A mutation in the KYNU gene leads to a deficiency in kynureninase. The enzyme is involved in various biochemical processes. If there is too little kynureninase in the body, the cells cannot synthesize vitamin B3 (also called nicotinic acid or niacin) as usual, and hypovitaminosis occurs. Signs of a deficiency in B3 include dermatitis and inflammation of the mouth, stomach and intestinal mucosa. In addition, diarrhea, depression, loss of appetite, difficulty concentrating, sleep disorders and irritability can occur. The deficiency can also trigger the pellagra disease.
It is not only the human organism that forms hydrolases. Pathogens such as bacteria can also produce enzymes from this group. An enzyme that can even harm people is called urease, and it breaks up urea into ammonia and carbon dioxide. The ammonia helps the bacteria to withstand stomach acid. As a result, they can infect the digestive system and cause a range of ailments. The Helicobacter pylori bacterium belongs to this group of pathogens. Helicobacter pylori triggers type B gastritis, can be responsible for gastric and duodenal ulcers and, in the case of chronic infection, cause gastric carcinoma.