Imatinib is a tyrosine kinase inhibitor that is mainly used to treat chronic myeloid leukemia. It achieves good results in the treatment of chronic myeloid leukemia with good tolerance at the same time. It can also be used for other malignant diseases.
What is imatinib?
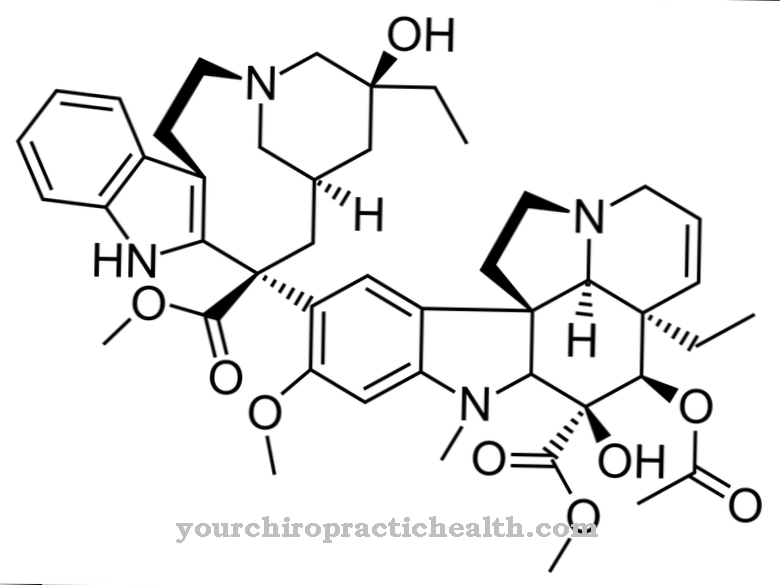
Imatinib (trade name Glivec®) is a drug from the group of tyrosine kinase inhibitors that is used to treat chronic myeloid leukemia, to treat malignant tumors of the gastrointestinal tract and to treat other malignant diseases. The chemical formula of Imatininb is C29H31N7O.
Pharmacological effect
Chronic myeloid leukemia is triggered by the so-called Philadelphia chromosome, a genetic change. The Philadelphia chromosome has a translocation of genetic material from chromosome 9 and chromosome 22. As a result of this translocation, the gene for the natural enzyme tyrokinase-ABL on chromosome 9 "fuses" with the fragment of the BCR gene on chromosome 22.
Instead of the ABL tyrosine kinase, the mutated cells produce a so-called BCR-ABL fusion protein. BCR-ABL is a more active tyrosine kinase compared to ABL. This BCR-ABL leads to the uncontrolled reproduction of white blood cells (leukocytes) and is significantly involved in the development of chronic myeloid leukemia.
Imatinib has an inhibiting effect on the activity of tyrosine kinase and thus suppresses the pathologically increased proliferation of mutated blood stem cells. The substance is administered orally in the form of a tablet; Imatinib mesilate, a salt, is used medicinally. The aim of treatment is to reduce the pathological cell clone as much as possible.
In more than 95% of the patients treated with imatinib who suffered from chronic myeloid leukemia, normalization of the blood count is achieved.
Medical application & use
As already mentioned, the substance is mainly used in the therapy of chronic myeloid leukemia. However, it is also effective against a number of other cancers. It is also indicated for acute lymphatic leukemia, hypereosinophilic syndrome, various skin tumors, malignant tumors of the gastrointestinal tract, aggressive mastocytosis and certain myeloproliferative diseases.
In chronic myeloid leukemia, a neoplastic disease of the hematopoietic system, more immature forms of leukocytes appear in the blood, which is due to the pathologically increased increase in leukocytes in the blood and in the blood-forming bone marrow.
Chronic myeloid leukemia results from a (genetic) disorder of the hematopoietic (blood-forming) stem cells found in the bone marrow. For this reason, chronic myeloid leukemia is one of the myeloproliferative neoplasms. The cause of the disease is the change and subsequent reproduction of a single multipotent hematopoietic progenitor cell. In almost all cases, this change is due to the Philadelphia chromosome described above.
The prognosis of chronic myeloid leukemia has been significantly improved thanks to the new drugs from the group of tyrosine kinase inhibitors, which also include imatinib. Therapy with tyrosine kinase inhibitors is a highly effective treatment option with relatively few side effects and is considered a targeted therapy.
The survival rate has increased greatly with the introduction of tyrosine kinase inhibitors. When there were no therapeutic options for chronic myeloid leukemia, the mean survival time of patients was between three and four years.
Chronic myeloid leukemia was the disease with the worst prognosis among the myeloproliferative neoplasms. With the introduction of hydroxycarbamide, a cytostatic, this mean survival time was increased to four and a half years. Interferon led to a further increase in the mean survival time to about five and a half years.
Treatment with tyrosine kinase inhibitors is now considered the standard therapy. The 5-year survival rate with imatinib treatment is over 90%. The follow-up time of patients treated with imatinib is now over 10 years, the "mean survival" has not yet been established. This suggests that it is very clearly above the mean survival of the previously used therapies (with hydroxycarbamide and interferon).
Risks & side effects
The imatinib is generally well tolerated. However, diarrhea, vomiting, abdominal pain, nausea, indigestion, fatigue, headache, edema, weight gain, muscle cramps, muscle pain, joint pain, rash, bone pain and changes in the blood count can occur.
Imatinib is only contraindicated in cases of hypersensitivity or intolerance to imatinib.
Imatinib should not be taken at the same time as paracetamol as it inhibits glucuronidation (binding to glucuronic acid during metabolism) of paracetamol. Furthermore, certain subunits of the cytochrome P450 are influenced, which can lead to interactions with other drugs.





















.jpg)



