Peptides are molecules whose amino acids are linked by peptide bonds. They take on numerous functions and, in addition to hormonal effects, can also have analgesic or anti-inflammatory effects. Because of their numerous tasks, peptides are now used as active ingredients in medicines.
What is a peptide?
Proteins are macromolecules made up of amino acids. The peptide can also be called a protein in this sense. Peptides contain peptide bonds of amino acids. These peptide bonds correspond to amide-like bonds between the α-carbon atom amino group and the carboxy group of two amino acids.
Peptides differ from real proteins in terms of their molar mass. The number of all linked amino acids in a peptide therefore does not equal the number of them in a protein. Nevertheless, there is a fluid demarcation between proteins and peptides. The term protein is usually used for amino groups made up of more than 100 amino acids. Anything below that is more likely to be referred to as a peptide. The term peptide was coined by Emil Fischer at the beginning of the 20th century.
He used the term based on peptone and thus the protein breakdown products of pepsin, adding the ending of polysaccharide to the new term because of the similarity to monomers. Peptides are systematically divided according to the number of amino acids. For example, oligopeptides contain fewer than ten amino acids. Polypeptides contain up to 100 and cyclopeptides contain at least two amino acids in a ring structure.
Function, effect & tasks
Peptides assume a wide range of physiological functions in the body, which differ with the type and structure of the peptide form. For example, they can have a hormone-like effect or have anti-inflammatory effects. They also have pro-inflammatory effects.
The same applies to antibiotic or antiviral functions. Some peptides regulate metabolic processes. Growth and the sensation of pain are also linked to peptides. All peptide substances in the body are highly active and have a highly specific activity. As calcitonin, they lower the calcium level in the thyroid, for example. As endorphins, they occur in many organs to reduce pain. As glucagon in the pancreas, they are involved in the breakdown of glucagen.
They are also found in the pancreas as insulin and are involved in glucose uptake. They are found in the parathyroid gland in the form of parathyroid hormone and mobilize calcium to build bones. As somasostatin, they also regulate growth processes and hormonal effects in various organs. They are just as important as neurotransmitters and vasilidators. Opioid peptides act like morphine and apparently support physical and mental development.
Education, occurrence, properties & optimal values
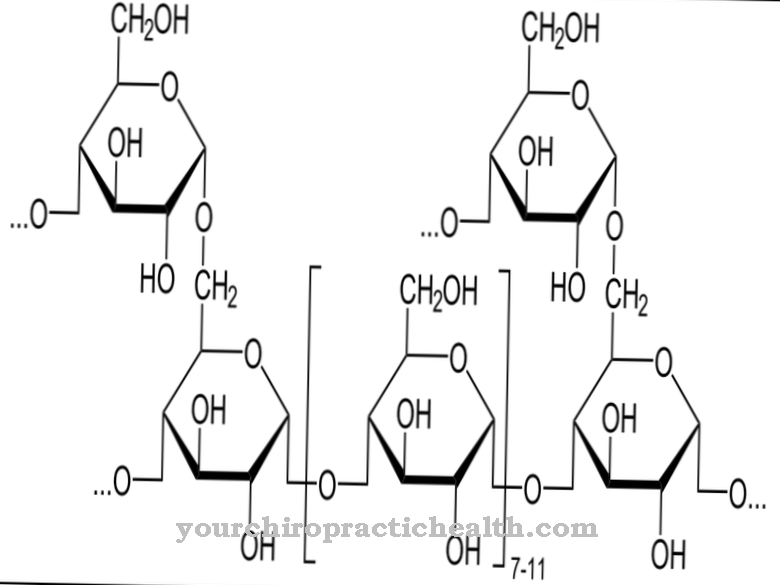
Amino acids are always arranged in a defined sequence in the peptide and usually correspond to a linear chain. The number of amino acids in a peptide is called the chain length. Depending on this chain length and arrangement, peptides are either olgio, poly or cyclo peptides. There are α-peptide bonds, ω-peptide bonds and isopeptides. Circularly linked peptides are cyclopeptides.
As part of the transcription, peptides are formed from α-amino acids in L-form. This process uses genetically encoded amino acids. Occasionally, D-amino acids can occur in peptides that occur as products of specific metabolic pathways and are therefore not biosynthetic products. The synthesis of polypeptide chains is carried out by ribosomes. In addition, there is the non-ribosomal peptide synthesis, which is carried out enzymatically by the peptide synthetases. Some dietary proteins are also processed into opioid peptides through digestive processes.
The carboxy group of an amino acid reacts during condensation with the escape of water with the amino group of another amino acid to form an acid amide group, which is also known as -CO-NH-. The amide bond between a nitrogen atom and a carbon atom of the carbonyl group in question is then transformed into a peptide bond. Peptide bonds are not free in their elasticity because there are two structures of resonance.
Diseases & Disorders
Modern medicine uses peptides as drugs for various diseases. The substances have been used, for example, in cancer therapy for many years.
In this context, peptides are currently used, for example, which appear as conjugates to cytotoxic molecules. These peptides detect tumors and deliver a cytostatic agent to the tumor in order to locally kill the cells. This therapy is a gentler and more locally applicable alternative to conventional chemotherapy. Peptides are also used in therapeutic vaccines. This therapy option is about the immunological presentation of antigen surfaces and their fragments.The immune system then forms specific antibodies to neutralize the antigen presented. Many antigens have a proteinogenic structure.
The reproduction of peptides and their use in vaccines is particularly suitable for this reason. Both against various epidemics and against allergies, peptides as vaccines can offer such a solution. As highly active, specific active ingredients, peptides are used in a wide variety of drugs. Thanks to their biologically diverse functions, they can be used in a wide variety of applications. They have also established themselves as drugs against diabetes and obesity. The same applies in connection with cardiovascular or neurodegenerative diseases, for organ insufficiencies or as an alternative to antibiotics. Oral administration is not particularly effective because of the rapid digestion.
Therefore, the drug is usually given parenterally. Injection and depot formulations with a duration of action lasting several days are now just as used as nasal applications. In view of their diverse modes of action, peptide-based diseases or mutations have a correspondingly wide variety of symptoms. Today, many of these diseases can be treated relatively well thanks to the advances in artificial peptide synthesis.

.jpg)
.jpg)





















