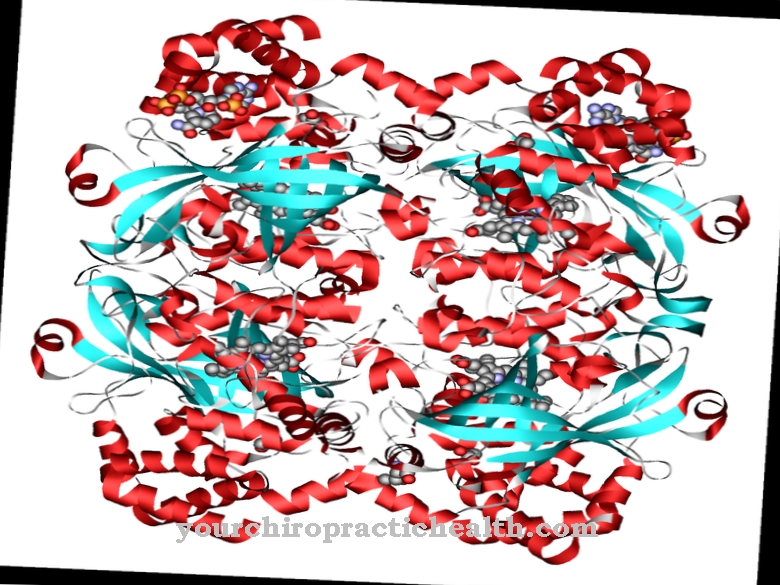
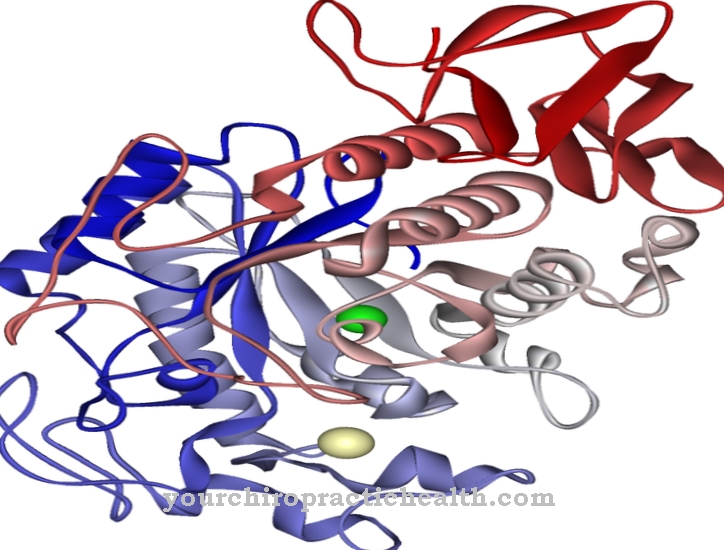
Peptidases are enzymes that catalytically break down peptide bonds of peptides and proteins through hydrolization, i.e. through the attachment of an H2O molecule.
Peptidases work extra- and intracellularly. It is not only about the breakdown of proteins and peptides for energy production and the extraction of fragments for the construction of new proteins, but also a number of specialized functions such as activation of enzymatically acting proteins or neurotransmitters.
What is a peptidase?
The most important characteristic that characterizes all peptidases is their ability to break peptide bonds between two amino acids via hydrolysis. An H2O molecule is deposited and practically corresponds to the reverse of the peptization process.
Proteins and peptides are made up of a string of amino acids via peptide bonds. The only difference between peptides and proteins is that proteins consist of chains of more than about 100 amino acids, while peptides have shorter chains of at least two to a maximum of 100 amino acids. The large number of peptidases specializing in certain points of attack and catalysis processes poses a challenge for a general classification. A basic distinction can be made between exo- and endopeptidases.
Exopeptidases attack peptide chains either from the N-terminal end (aminopeptidases) or from the C-terminal end (carboxypeptidases) and are specialized in splitting off one amino group at a time or whole peptide fragments with two, three or more amino acids. Endopeptidases specialize in attacking specific points on proteins.Often this is the process of activating an enzyme, comparable to removing a locking lever. Peptidases can be classified according to the international EC nomenclature.
Function, effect & tasks
Peptidases fulfill several different basic functions. The most noticeable function and task in the catabolic branch of the metabolism consists in the fragmentation of proteins in food in order to be able to absorb them through the intestinal mucosa.
The protein fragmentation function is also required within the body to break down the body's own proteins for the purpose of generating energy or for the production of protein fragments for the anabolic part of the metabolism to build up new proteins. Another function and task is to activate certain proteins after synthesis by splitting off a certain amino acid. So-called signal peptidases detach signal peptides from proteins. This ensures that proteins produced intracellularly are transported to their intended place of use. An important task for peptidases is their participation in the synthesis of antigens.
To perform this function, peptidases combine to form a peptidase complex, the proteasome. Intracellular peptidases can be found in almost all cell compartments and cell organelles, where, among other things, they maintain the protein balance. Peptidases also take on important functions within the coagulation process and are therefore jointly responsible for the rapid closure of a wound without blood clots forming, which can be carried away with the bloodstream and lead to heart attacks.
Education, occurrence, properties & optimal values
Peptidases occur in almost all tissues as extracellular peptidases and in practically all cells as intracellular peptides, each with different tasks but similar functions and effects. Extracellular peptidases are secreted by exocrine glands such as the salivary glands in the mouth, the gastric mucosa and, above all, by the pancreas.
The process of protein breakdown begins in the mouth and continues in the stomach. The final breakdown of the proteins into defined pieces, which can be taken up and resorbed by the endothelial cells of the intestinal mucosa, occurs mainly in the duodenum, the first section of the small intestine after the stomach. In the small intestine, preliminary stages of digestive enzymes, called zymogens, are activated by peptidases in the bioactive form by splitting off certain amino acids. The specification of reference values or optimal concentrations of peptidases can only ever relate to a specific peptidase and, at best, only allows conclusions to be drawn about the existence of certain problems by comparing them with some other laboratory parameters and subsequent differential diagnosis.
For example, an elevated leucine-amino-peptidase (LAP) level cannot yet conclude that cholestasis, a build-up of digestive enzymes, is present. The LAP values for women are 16-32 units per liter and for men 11 to 35 units per liter. If the levels are excessively high, they should be checked with the levels of certain liver enzymes in the blood such as alkaline phosphatase, gamma-GTP and some other values.
Diseases & Disorders
Enzymes represent the largest group of substances within proteins. Peptidases and lipases play an important role as digestive enzymes.
The large number of peptidases - more than 250 different peptidases are known - mean that metabolic disorders that have been acquired, i.e. caused by an unbalanced diet, illness or toxins, can also occur. On the other hand, it can be assumed that the complex interplay of enzyme metabolism can also be influenced and impaired by gene mutations. The symptoms and risks that result from the metabolic disorders can range from mild to severe.
It is only recently that the connections between non-specific symptoms and specific disorders in the metabolism of peptidases and other enzymes have been researched. A reduced peptidase activity in the intestine leads to an increased uptake of longer-chain peptides, which arise as fragments of proteins in the blood and to an increased excretion via the kidneys, so that the facts can be determined relatively easily by a urinalysis. Interestingly, decreased peptidase activity has been linked to diseases such as ADD, ADHD, schizophrenia, autism, and depression.