Plasmin is a protein-splitting enzyme in human blood serum, which is formed from the precursor plasminogen. Its main task is fibrinolysis and thus the body's own breakdown of blood clots. Overactivity of plasmin can lead to a tendency to bleeding and underactivity to a tendency to thrombus.
What is plasmin
The human blood serum contains various proteins and enzymes. Enzymes consist of giant biological molecules and act as catalysts to accelerate chemical reactions. Almost all enzymes in human blood are proteins that are formed by the protein biosynthesis of the ribosomes.
The enzymes have a wide variety of tasks in the organism. Depending on their function, they are further classified. The peptidases are, for example, a group of enzymes that cleave peptides or proteins. In this way, they catalyze the hydrolysis of peptide compounds. Peptidases are also called proteolytic enzymes. One such proteolytic enzyme is plasmin. It occurs in the blood serum and breaks down various proteins there. The breakdown of proteins from the serum also falls within his area of responsibility. Plasmin is formed from the precursor plasminogen.
Function, effect & tasks
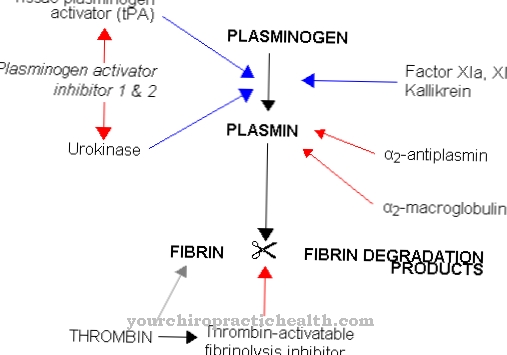
The main function of plasmin is to break down fibrin. This so-called fibrinolysis plays a role especially for blood clots. During this process, the plasmin dissolves the body's own blood clots by breaking down the fibrin polymers of the thrombus into fibrin breakdown products.
Fibrinolysis is regulated by counteracting biochemical processes. Activation takes place by converting the inactive plasminogen into active plasmin. Fibrinolysis works together with blood clotting, but progresses significantly more slowly. Two endogenous activators are involved in the activation of fibrinolysis: the tissue-specific plasminogen activator and urokinase. As non-physiological activators, staphylokinase and streptokinase are involved in the activation of plasmin.
The exogenous activators form a larger complex with plasminogen and plasmin, which activates the inactive plasminogen. PAI-1 to PAI-4 appear as inhibitors of fibrinolysis activation. After activation, the plasmin cleaves the fibrin polymers. It binds to fibrin and separates the branched fibrin polymers into soluble degradation products with different structure and mass. The blood circulation carries away the soluble substances until they are flushed out of the bloodstream.
To deactivate fibrinolysis, the body uses the plasmin inhibitor alpha-2 plasmin inhibitor. Fibrin-bound plasmin has a relatively long half-life compared to this antiplasmin. Free plasmin in the serum is rendered harmless in a very short time by the inhibitor.
Plasmin thus takes on important tasks in the coagulation system and appears as an opponent of thrombin. In addition to the fibrin, the preliminary stage fibrinogen is also broken down by plasmin and its preliminary stage. Serine proteases like plasmin have an irreversible effect and do not catalyze biochemical reactions in both directions. Plasmin has an autocatalytic effect and converts other molecules into active plasmin.
Its proenzyme is therefore a substrate for the activated one. Aside from its fibrinolytic activity, plasmin also breaks down proteins such as activated collagenases. In addition, it activates various mediators in the complement system and thins the wall of Graaf's follicles during ovulation.
Education, occurrence, properties & optimal values
Plasmin is produced from the preliminary stage plasminogen. It is synthesized in the liver and then released into the bloodstream, where it can be measured. Plasminogen has a half-life of more than two days. Free plasmin can hardly or not at all be detected in the blood. Only plasminogen can be determined. The determination usually takes place in the citrated blood. The normal values for plasminogen activity are between 85 and 110 percent. The standard value for the plasminogen concentration is 0.2 g per liter.
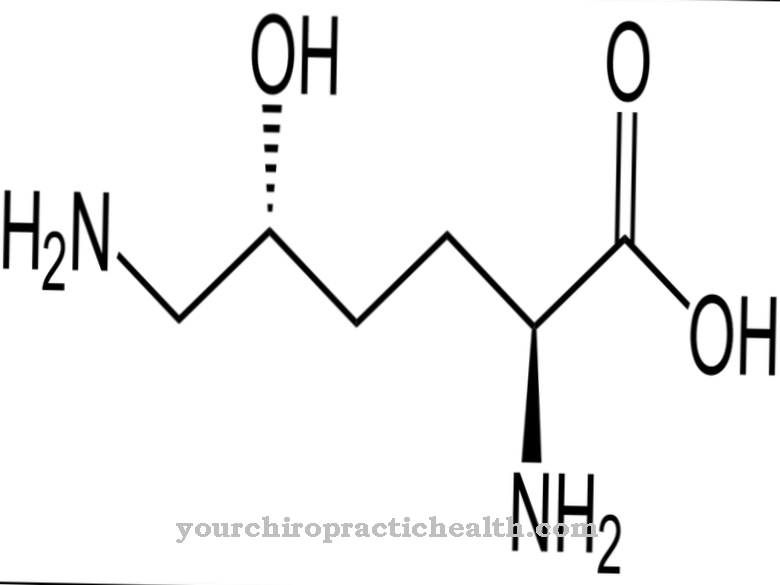
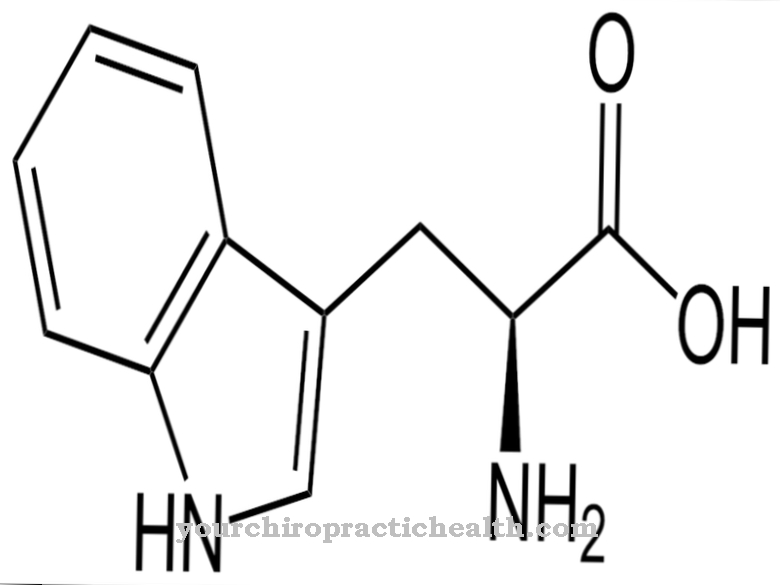
Plasminogen becomes plasmin, which, like elastase and trypsin, corresponds to an endopeptidase. The activation of plasminogen to plasmin takes place through various substances. The most important are tPA, thrombin, factor XII and fibrin. As part of the peptidase subgroup serine protease, plasmin has an active center. In this active center, serine proteases carry a catalytic triad in which the amino acid serine is involved. The catalytic triad of aspartic acid, histidine and serine contains amino acid residues that are linked by hydrogen bonds.
Diseases & Disorders
A plasmin-related disease is plasminogen activator inhibitor 1 deficiency. This congenital deficiency leads to the premature dissolution of blood clots, which manifests itself in a tendency to bleed.
PAI-1 appears in the healthy body as an inhibitor of the tissue type plasminogen activator, as it plays a role in intravascular fibrinolysis. Spontaneous bleeding is rarely a symptom of the disease. Nonetheless, minor trauma can cause bleeding to the knees, elbows, nose or gums. Menstrual bleeding is often increased. Long periods of bleeding after surgery are often observed in patients. If there is only a partial deficiency of inhibitors, bleeding occurs less frequently. There may also be no bleeding at all or only slight bleeding.
In some patients, the inhibitory protein is present but not working. The cause is a mutation of the associated alleles. The disease of the homozygous state is based on an autosomal recessive inheritance.The ELISA antibody test or the analysis of the PAI-1 function enables the diagnosis to be made. Fibrinolysis inhibitors such as epsilon-amino-caproic acid or tranexamic acid are administered to the patient as a countermeasure to prevent bleeding.
A mutation-related decreased activity of plasmin is the opposite of the disease described and can promote a thrombotic tendency. Modern medicine also assumes that the breakdown of connective tissue by the enzyme plasmin plays an important role in the spread of various diseases. Associated diseases now include cancer, cardiovascular diseases and inflammation.





.jpg)

.jpg)


.jpg)