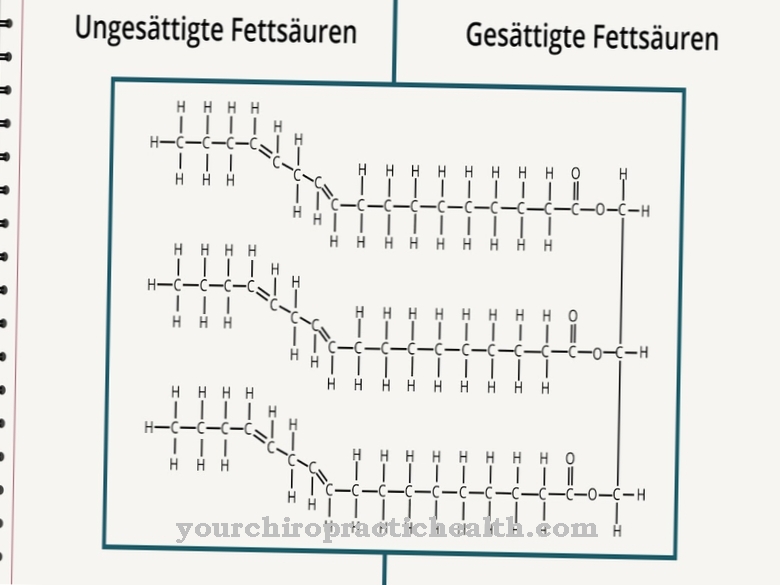
Hydroxylysine is a non-classical proteinogenic amino acid. It is incorporated into the corresponding protein as lysine and hydrolyzed to hydroxylysine within the polypeptide with the help of an enzyme. It is one of the main components of the collagen proteins in connective tissue.
What is Hydroxylysine?
Hydroxylysine is a proteinogenic amino acid that is first incorporated into a protein as lysine. Therefore it is a non-canonical proteinogenic amino acid. The term "canonical" means classical.
So there is no codon for this amino acid. Hydroxylysine is mainly found in connective tissue collagen and in glycoproteins. There, lysine is converted into hydroxylysine via enzymatic processes. Only part of the lysine is converted into hydroxylysine. The properties of the respective collagens depend on the amount of hydrolyzed lysine and proline residues.
Hydroxylysine can be isolated in free form as the hydrochloride. The hydrochloride of hydroxylysine is a beige powder with a melting point that is 225 to 230 degrees. It is a basic amino acid that also makes hydroxylysine-containing proteins react basic. Hydroxylysine was discovered by the American biochemist and co-founder of "Clinical Chemistry" Donald Van Slyke (1883-1971).
Function, effect & tasks
Hydroxylysine is of great importance for the structure of the connective tissue. Glycoproteins also contain hydroxylysine in order to form glycosidic compounds of the protein with sugar residues on the hydroxyl residue.
Within the collagen, it is responsible for the cross-linking of the individual protein molecules. Together with hydroxylproline, the hydrolyzed form of proline, it is also instrumental in building the tertiary and quaternary structures of collagen. The hydroxylation of lysine is catalyzed by the enzyme lysyl hydroxylase with the participation of the cofactors iron ions and ascorbic acid (vitamin C). The distribution pattern of the hydroxylated lysine residues in collagen is neither particularly rigid nor flexible. There are always recurring patterns.
However, there are also entire areas within the protein that do not contain any hydroxylated lysine residues. While hydroxyproline is responsible for the helical structure of the collagen through the binding of three protein chains, cross-links between the various protein molecules are formed via the hydroxyl groups of the hydroxylysine. In addition, these molecular groups also serve as a binding site for a glycosidic bond with a sugar. Overall, this ensures the strength of the connective tissue.
If there is a deficiency in hydroxylysine within the proteins, this cannot be remedied by an additional intake of the amino acid. There is no codon for free hydroxylysine, so that it cannot be incorporated into the corresponding protein. The value of dietary supplements with added hydroxylysine is therefore very questionable. Therefore, the deficiency must be due to insufficient hydroxylation of lysine.
Education, occurrence, properties & optimal values
Hydroxylysine is only found in human and animal collagen. There are also some glycoproteins that also contain hydroxylysine. This includes adiponectin. Adiponectin is a hormone that is produced in adipose tissue and has a decisive influence on the effectiveness of insulin. Hydroxylysine has also been detected in some bacteria, such as Staphylococcus aureus.
The distribution of the hydroxylated lysine is not uniform in the collagen. There are locations where it is almost always found. In other areas, hydroxylysine is almost never found. This uneven distribution determines the structure of the collagen. Within the triple helix structure of collagen, hydroxylysine is always located at the Y position of the repeating sequence Gly-X-Y. In the short regions with a non-helical structure, hydroxylysine also occurs in other places.
Diseases & Disorders
The connective tissue is absolutely dependent on the presence of hydroxylysine. Collagen can only be stable and firm if the cross-links between the protein molecules work. A deficiency in hydroxylysine causes connective tissue weakness.
If it is only present in extremely small amounts or not at all, the corresponding organism would not be viable. The connective tissue could no longer perform its task as a limiting and supporting tissue for the organs. In fact, there are diseases that can be traced back to a lack of hydroxylysine. Since this amino acid is initially incorporated as lysine during protein synthesis, it cannot be a primary deficiency. Hydroxylysine is formed from lysine within the collagen protein with the help of lysyl hydroxylases. A hydroxylysine deficiency can only result from a defect in this enzyme or its insufficient function.
There is a group of heterogeneous congenital connective tissue weaknesses that is known as Ehlers-Danlos syndrome. A number of mutations can be responsible for this clinical picture. Among other things, the lysyl hydroxylase can also be defective, so that too little lysine is hydroxylated. The Ehlers-Danlos syndrome manifests itself through overstretchability of the skin and overmobility of the joints. Internal organs, blood vessels, tendons, ligaments and muscles are also affected. The prognosis depends on the severity of the defect. If the vessels are involved, an unfavorable course is to be expected. The complete failure of the enzyme lysyl hydroxylase is incompatible with life and is therefore not observed.
But even with an intact enzyme, there may be weak connective tissue due to its low activity. Lysyl hydroxylase requires iron ions and ascorbic acid (vitamin C) as cofactors. If, for example, vitamin C is missing, what is known as scurvy occurs. Scurvy is an acquired connective tissue disease caused by a lack of hydroxyl groups on the proline and lysine residues of collagen. The cause is the low activity of proline hydroxylase and lysine hydroxylase due to the ascorbic acid deficiency.