Prostacyclin is a tissue hormone that belongs to the series 2 prostaglandins. The hormone is mainly produced in endothelial cells of the blood vessels and in cells of the smooth muscles from arachidonic acid.
It has a local vasodilator effect, increases pain by sensitizing the nociceptors, triggers fever and to a large extent inhibits platelet aggregation.
What is prostacyclin?
Prostacyclin, also known as prostaglandin 12 or PGl2 for short, belongs to the group of five tissue hormones of the series 2 prostaglandins. The hormone only occurs in animal cells, not in plant cells.
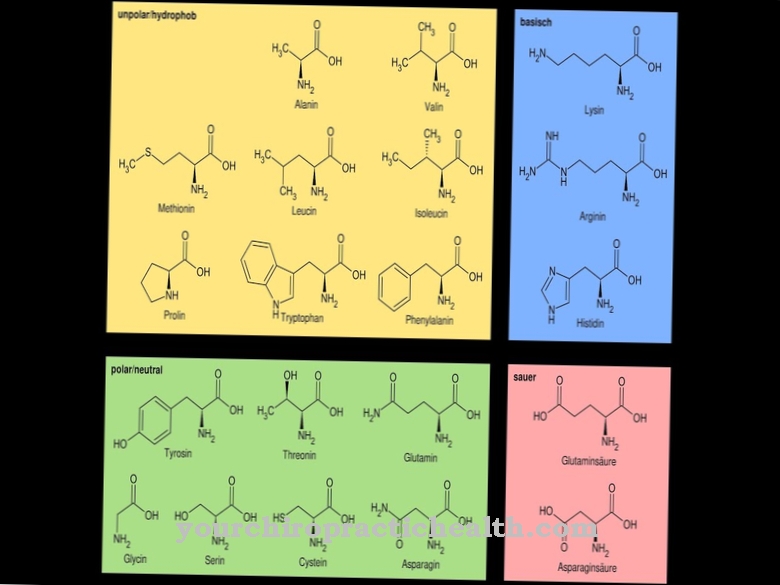
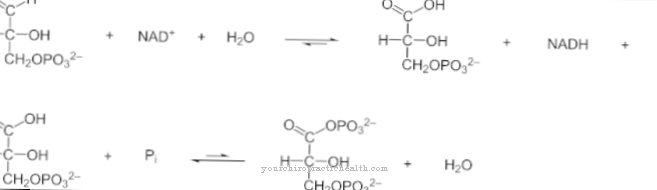
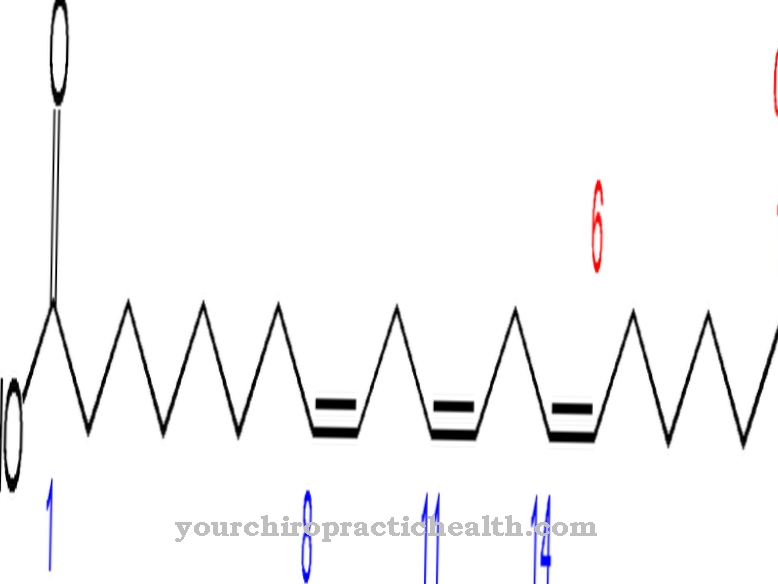
The synthesis of prostaglandins is closely linked to lipid metabolism. Various fatty acids, each with 20 carbon atoms, are produced by dehydrating and lengthening the carbon chains. Arachidonic acid, one of the newly formed fatty acids, is four times unsaturated and the starting material for prostacyclin. The body's own synthesis takes place mainly in the endothelial cells of the vessels and in cells of the smooth muscles. The chemical formula of procyclin is C20H32O5.
It shows that the hormone is composed only of the three elements carbon, hydrogen and oxygen. With the exception of prostaglandin F2, in which 34 instead of 32 hydrogen atoms are bonded, all five Series 2 prostaglandins have the same chemical molecular formula. The sometimes very different enzymatic effect is due to the slightly changed tertiary structure of the compounds.
Function, effect & tasks
Series 2 prostaglandins act largely as antagonists of series 1 prostaglandins, which have anti-inflammatory and anticoagulant effects. Series 2 prostaglandins, on the other hand, increase inflammatory reactions, constrict blood vessels and increase blood clotting processes.
In addition, they sensitize nociceptors so that pain sensations are perceived more strongly. One of the main tasks of prostacyclin, which is counted among the series 2 prostaglandins, is, together with prostaglandin E2, to stimulate the body, e.g. B. In the case of injuries, to induce local inflammatory reactions and to ensure an increased pain sensation. The hormone docks with so-called IP receptors, G-protein-coupled membrane receptors that specialize in prostacyclin, and induces the cell to react in certain ways via the receptor. The vascular permeability is increased, which leads to tissue swelling.
An externally visible reddening is based on the increased blood flow to the tissue in the injured area, which triggered the reactions. The intensification of pain comes about through an increased sensitization of the nerve endings of the nociceptors. A very important task of procyclin, which is synthesized in all endothelial cells of the vessels, is to prevent vascular contraction. This happens through the increased formation of cyclic adenosine monophosphate (cAMP), which is considered an antagonist of the thromboxane formed in platelets.
Prostacyclin is considered to be the most powerful endogenous platelet inhibitor due to the effective inhibition of thromboxane. The hormone also inhibits the so-called MAP kinase pathway, which includes multi-level signal transduction pathways. MAP kinase is involved in cell differentiation, in embryogenesis and in apoptosis, the programmed cell death.
Education, occurrence, properties & optimal values
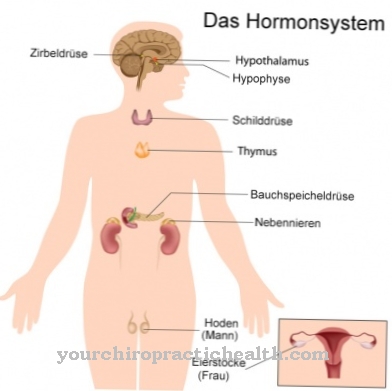
Prostacyclin is almost ubiquitous in almost all types of human tissue and is mainly synthesized in the endothelial cells, which form a single cell layer as squamous epithelium, the innermost layer of the walls of blood and lymph vessels. The number of endothelial cells in humans is an unimaginable 10,000 billion, and the cells are in contact with the blood over a total area of 4,000 to 7,000 square meters.
In the endothelial cells, the enzyme prostacyclin synthase catalyzes prostacyclin from arachidonic acid via the intermediate prostaglandin PGH2. Prostacyclin synthase is found in humans as a membrane protein in the endoplasmic reticulum of the cells of almost all types of tissue. Arachidonic acid, the starting substance for prostacyclin, is found in many foods of animal origin. Their proportion in pork lard is particularly high at 1,700 milligrams per 100 grams.
The hormone is subject to rapid bio-catalytic-enzymatic reactions with a half-life of only 3 minutes, and the concentration can, depending on the situation, rise sharply to 15-20 times the normal value within minutes, e.g. B. during general anesthesia during operations. The specification of an optimal concentration or the specification of reference values is therefore not appropriate.
Diseases & Disorders
In the lipid metabolism, various disorders of the synthesis can occur. If the two essential omega-6 and omega-3 fatty acids are missing in the metabolism, series 1 and series 3 prostaglandins cannot be synthesized, but series 2 prostaglandins, including prostacyclin, can.
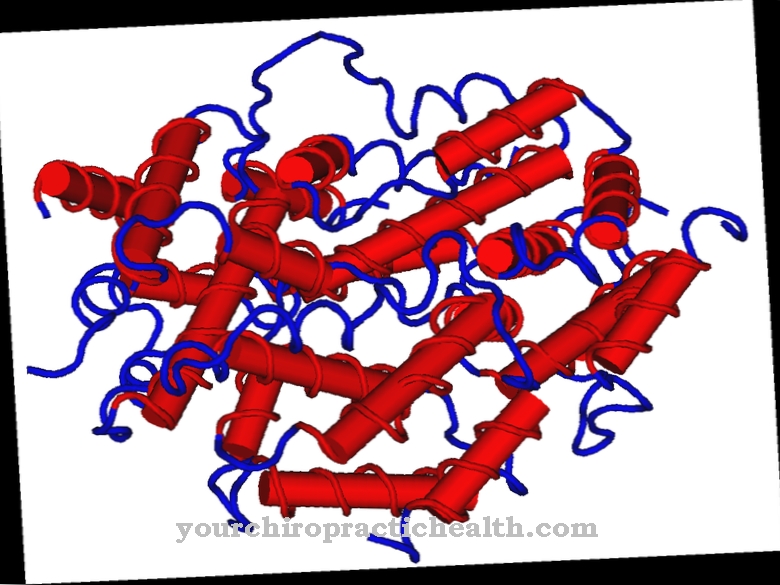
The two cyclooxygenases, COX-1 and COX-2, play an essential role here. Both enzymes are expressed by different genes, and both enzymes have different roles. The protein structures of COX-1 and COX-2 cyclooxygenases could only be sequenced in the 1990s. It was also only recognized towards the end of the 1990s that the synthesis of prostaglandins can be controlled via the availability of COX-1 and COX-2. The two cyclooxygenases are globular proteins with approx. 600 amino acids, the bio-active centers of which are almost identical despite different physiological properties.
If the prostacyclin synthesis is too low, symptoms such as increased tendency to thrombosis and circulatory disorders tend to be unspecific. For example, the very rare and hereditary Hermansky-Pudlack syndrome is associated with a pathologically reduced prostacyclin synthesis. The disease is characterized by ocular albinism and impaired platelet aggregation. Prostacyclin and its analogues used for the treatment of diseases. First and foremost, ischemic events are to be mentioned that arise due to arteriosclerotic occlusions or vascular obstruction.
For example, the very rare Raynaud's syndrome, also known as white finger disease, can be treated with prostacyclin to cover up the spastic narrowing of the vessels on the fingers or toes with the vasodilating properties of the tissue hormone.








.jpg)




.jpg)