Stearic acid In addition to palmitic acid, it is a main component of fats and oils. It is an unsaturated fatty acid with 18 carbon atoms whose main function is to store energy. Since it can be synthesized in the organism, it does not have to be taken in with food.
What is stearic acid?
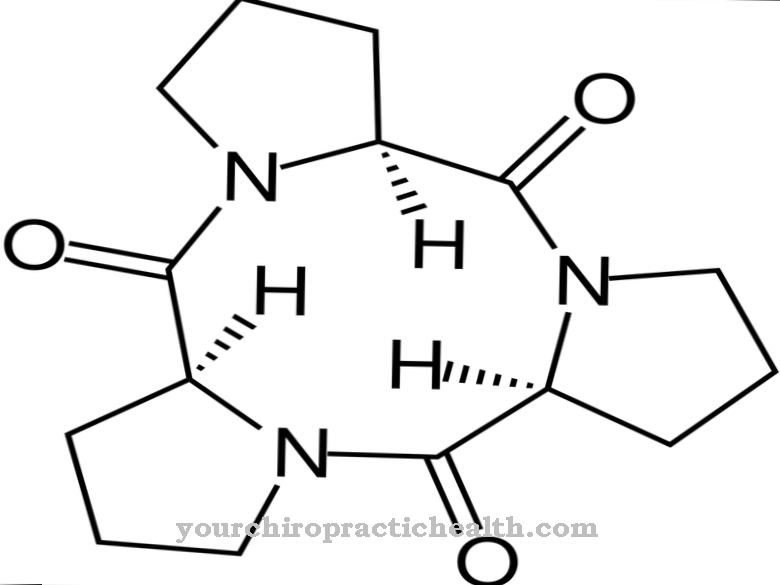
Stearic acid and palmitic acid are the two main components in vegetable oils and animal fats. Stearic acid consists of 18 carbon atoms. It is therefore also known as octadecanoic acid. As with palmitic acid, the chemical structure is very simple.
The 17-carbon hydrocarbon chain has a carboxyl group at one end. The carboxyl group provides the acidic properties of the molecule. Due to the long hydrocarbon chain, the compound is almost insoluble in water. In free form it is a white, tasteless solid that melts at 69 degrees and boils at 370 degrees. The salts of stearic acid are called stearates. Stearic acid and palmitic acid have similar chemical and physical properties.
They only differ in the length of the hydrocarbon chain, which in the case of palmitic acid is only two carbon atoms shorter. Both fatty acids also determine the properties of the triglycerides (fats and oils). While palmitic acid occurs in high concentrations in both animal and vegetable fats and oils, stearic acid is mainly contained in animal fats. Vegetable oils usually only contain up to a maximum of 7 percent stearic acid.
In addition to the triglycerides, stearic acid is also found in cell membranes and nerve fibers. There it is present as a phospholipid or sphingolipid. Due to their chemical structure, which is similar to that of palmitic acid, both fatty acids are always associated. In the animal or human organism, stearic acid is produced from palmitic acid by the addition of two carbon atoms.
Function, effect & tasks
The biochemical structure of stearic acid is not spectacular. Nevertheless, it is of great physiological importance. As already mentioned, stearic acid is a rather simply built hydrocarbon chain with a carboxyl group. In the organism it is bound to glycerine and serves as an effective energy store.
Burning 100 grams of stearic acid releases approximately 900 kilocalories. That's almost double the energy of the same amount of carbohydrates. The hydrocarbon bonds, which are found in large numbers in long-chain fatty acids, are particularly high in energy. Because of this energy storage capacity, stearic acid and the other fatty acids are effective as energy stores in the body. For this purpose, three more fatty acids are esterified with a glycerine molecule to triglycerides or fats and oils. These triglycerides compress the energy-rich molecules again in a very small space, so that the fats can function as one of the most energy-rich energy storage molecules.
In evolution, organisms have developed which, by storing fats and oils, have found a way to be able to provide for bad times. Among other things, stearic acid and palmitic acid are also the starting materials for the synthesis of the more biologically active unsaturated fatty acids. Many active ingredients such as prostaglandins can be formed on their basis. According to previous knowledge, stearic acid alone does not have any major physiological effects.
In addition to its function as an energy store, it is also a main component of phospholipids and sphingolipids, which in turn determine the structure of the cell membranes and the membranes of the cell organelles. The molecules, consisting of hydrophilic and hydrophobic components, separate the cells from the intercellular area. The hydrophobic fatty acid chains protrude from the membrane towards the cytoplasm of the cell. At the same time, the hydrophilic part of the cell points towards the cell surface. More recent research results indicate a further physiological effect of stearic acid.
Scientists from the German Cancer Research Center discovered by chance that stearic acid could have a controlling effect on mitochondria. The stearic acid molecule acts as a signal transmitter and leads to the fusion of mitochondria. As a result, mitochondrial function improves. Stearic acid could therefore be used in the future for therapy in mitochondrial diseases.
Education, occurrence, properties & optimal values
Like all other fatty acids, stearic acid is synthesized by building up a hydrocarbon chain through the gradual addition of two carbon atoms. The starting compounds are mostly carbohydrates. However, the fatty acids and amino acids contained in food also serve as the basis for building higher-chain fatty acids. Animal fats contain a particularly large amount of stearic acid.
Beef tallow, mutton fat, butter fat and lard are very rich in stearic acid. Cocoa butter is the largest supplier of stearic acid from plant sources. Other vegetable oils and fats usually only have a maximum share of 7 percent. Free stearic acid is made by saponifying fats with boiling caustic soda. The first result is the sodium salt of the fatty acids, which are converted back into fatty acids through treatment with mineral acids.
The subsequent separation of the individual fatty acids is carried out using special physical (distillation) or chemical processes. Stearic acid is used in cosmetic products, shaving foam, cleaning agents and detergents.
Diseases & Disorders
Stearic acid has no harmful effect under normal conditions. It is toxic neutral and well tolerated. However, fine dust and vapors with stearic acid can have a caustic effect. This leads to local irritation, gastrointestinal problems and sometimes vomiting.
If the contact with these dusts and vapors is very intense, it can lead to respiratory problems and pulmonary edema. Another problem is magnesium stearate. It is produced industrially by the hydrogenation of palm oil, which, however, is contaminated with pesticides. Therefore, the magnesium stearate used in dietary supplements can have toxic effects on the liver. In addition, the use of magnesium stearate can cause skin damage and intestinal disorders.




.jpg)

.jpg)


.jpg)