The Xanthine oxidase is responsible for the conversion into uric acid during the breakdown of the purines. A deficiency or an inhibition of the enzyme leads to a decrease in uric acid levels in the blood.
What is xanthine oxidase?
Xanthine oxidase is an enzyme that initiates (catalyzes) the reaction of hypoxanthine via xanthine to uric acid. It contains a non-protein part as a so-called prosthetic (Greek, prosthetos, appended) group, which is responsible for reactivity.
It is a derivative of flavin. Xanthine oxidase is one of the flavin enzymes. It also has iron and molybdenum in the active center. In 1902 the enzyme was described for the first time as a component of cow's milk by the biochemist F. Schardinger, so that it was previously called the Schardinger enzyme. Under the action of this enzyme, the color of the dye methylene blue changes, which can be used as a typical detection reaction to differentiate between raw and heat-treated milk.
The enzyme is destroyed at high temperatures. The change in color is due to the fact that the enzymes present in raw milk (such as xanthine oxidase) in the presence of formaldehyde decolorize the methylene blue when exposed to air.
Function, effect & tasks
The best-known task of xanthine oxidase is the formation of uric acid within the purine metabolism. Purines are present in every cell. They are part of the nucleic bases adenosine and guanine, from which, together with the pyrimidine bases cytosine and thymine (or uracil), the nucleic acids DNA and RNA are built up.
The genetic information in the different cells of the body consists of nucleic acids. This applies to humans and animals. For example, people with meat products ingest a large amount in the form of animal foods. If the body's own cells go under or if animal cells that enter the body through food are broken down, nucleic acids and thus also purines are produced. These are converted into uric acid. Under the action of xanthine oxidase, hypoxanthine is first formed and, in a further step, xanthine, which is converted into uric acid and can largely be excreted via the kidneys. Only a small proportion of uric acid leaves the body through the intestines.
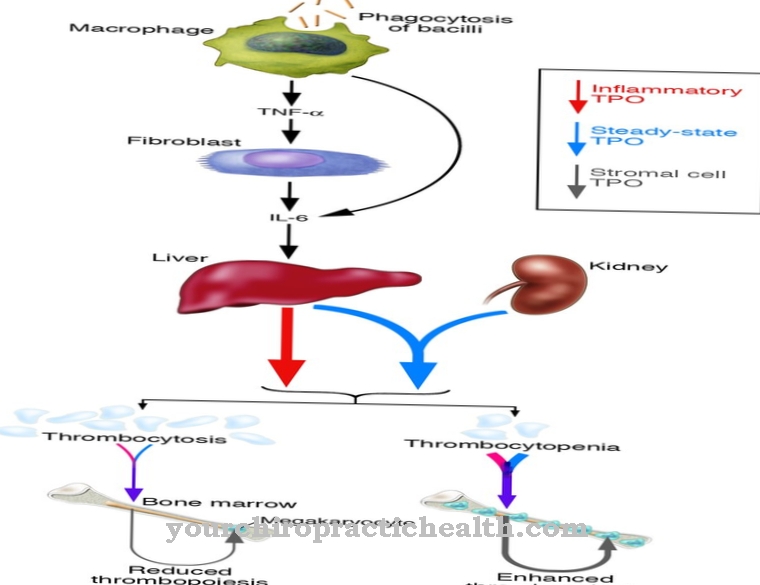
If the body accumulates large amounts of purine or if the excretion in the urine is restricted, the uric acid level in the blood rises. A second enzyme involved in purine breakdown also produces uric acid. It is called xanthine dehydrogenase, uses nicotinamide adenine dinucleotide (NAD), and is the predominant enzyme. In contrast, xanthine oxidase has a flavin adenine dinucleotide subunit (FAD). In the event of insufficient supply, for example due to a lack of blood flow, both enzymes can convert into one another.
The reaction under the action of xanthine oxidase also produces hydrogen peroxide as a by-product. Hydrogen peroxide is not itself a radical, but it is a reactive substance. This is why it is quickly rendered harmless by the action of other enzymes (peroxidase, catalase) in the body.
Education, occurrence, properties & optimal values
Purine breakdown takes place mainly in the liver. In the mucous membrane of the small intestine, the enzyme is also involved in the incorporation of iron into the transport protein transferrin. Research has shown that the enzyme is found in cells that are located in the inner wall of blood vessels. In this context, its influence on the course of cardiovascular diseases and damage caused by oxidative stress is discussed.
The proportion of oxidase in relation to dehydrogenase, the enzyme system responsible for converting purines to uric acid, is 20 percent. The xanthine dehydrogenase form is 80 percent present. The flavin contained in xanthine oxidase is one of the riboflavins, which is identical to vitamin B2. The molybdenum subunit contained in xanthine oxidase is bound by allopurinol, which is very similar in structure to purines. In this way the enzyme is almost completely inhibited. The activity of the enzyme can be determined indirectly from the amount of uric acid formed.
Diseases & Disorders
With meals rich in purine or with increased cell death, which occurs, for example, during cancer therapy, the xanthine oxidase reaction is activated and larger amounts of uric acid are produced. The level of uric acid in the blood increases.
If the enzyme is inhibited, the uric acid concentration in the blood drops. This effect is the basis of the medication in gout disease. In gout, the increased uric acid concentrations lead to crystallization and thus to discomfort in the joints. Drugs containing allopurinol are standard preparations used to treat gout. In the hereditary form of enzyme deficiency, the activity is reduced due to mutations. This condition is inherited as an autosomal recessive trait. The child becomes ill if both parents carry an affected allele. A reduced activity of xanthine oxidase also occurs when the molybdenum cofactor is deficient because it is not formed due to a defect.
Xanthine and hypoxanthine accumulate. Significantly increased xanthine levels in the blood and the appearance of xanthine in the urine (xanthineuria) are noticeable in those affected. When determining xanthine for the detection of enzyme deficiency, foods such as coffee beans, tea, mate or potatoes must be excluded as a source of xanthine. The uric acid levels, on the other hand, are lowered. Since xanthine is not broken down and is sparingly soluble, it can form crystals. In most cases the disease is symptom-free. However, others can have kidney problems, urinary tract infections, or blood in the urine.
If xanthine stones form in severe disease, they can be deposited in the kidney tissue or in the urinary tract. Those affected have to pay attention to a low-purine diet and plenty of fluids.





.jpg)