Valproic acid is a non-naturally occurring carboxylic acid. It was first synthesized in 1881 and is used as an anti-epileptic. It must not be used in pregnant and breastfeeding women.
What is valproic acid?
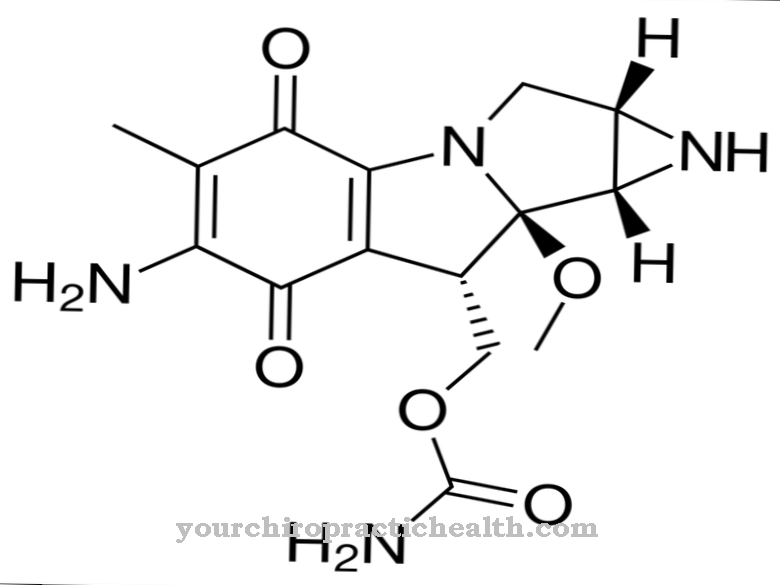
Valproic acid is a non-naturally occurring carboxylic acid. Carboxylic acids are organic compounds that have one or more carboxy groups (-COOH). Valproic acid and its salts (the so-called valproates) are used medicinally as antiepileptic drugs (anticonvulsants). The chemical formula of valproic acid is C8H16O2, its molar mass is 144.21 g · mol − 1.
Valproic acid was first synthesized in 1881. At first it was used as a solvent for water-insoluble substances. The synthesis of valproic acid takes place via the starting materials ethyl cyanoacetate and two equivalents of 1-bromopropane. When sodium ethoxide is added, these substances react via an anion of the enol form of the carbonyl compound to form the α, α-dipropylcyanoacetic acid ester. Ester cleavage and decarboxylation then take place in a basic environment.
These processes produce dipropylacetonitrile, which can be converted into valproic acid through a reaction with water (hydrolysis). The malonic ester synthesis is an alternative to the synthesis of valproic acid described above.
Pharmacological effect
In the therapy of epilepsy, valproates, the salts of valproic acid, are used, which are converted into valproic acid in the stomach. The application can take place either orally or intravenously.
Valproic acid is absorbed very quickly and there is also a plasma protein binding of over 90%. Valproic acid is metabolized in the liver; less than 3% of the active substance is excreted unchanged in the urine. The plasma half-life of valproic acid is 14 hours. However, it should be noted that it can decrease in combination with other epileptics.
The effect of valproic acid is due to the ability to close ion channels in the central nervous system.By closing the ion channels, the ions can no longer get into the cells and cannot trigger any action potentials there. Both sodium and calcium ion channels are affected by this effect of valproic acid. These two ion channels are responsible for the increased occurrence of action potentials in epilepsy.
Valproic acid also enhances the effect of the neurotransmitter GABA by inhibiting the breakdown of GABA and at the same time stimulating the synthesis of GABA. The neurotransmitter GABA leads to an increased influx of chloride ions into the cell, which leads to a reduced excitability of the cell.
In addition, valproic acid intervenes in the epigenetic system through acetylation, which can change cells and the activity of individual genes. Valproic acid inhibits the enzyme histone deacetylase and thus loosens the density of the DNA packaging. Valproic acid modulates gene activity via the degree of acetylation of histones. This mechanism leads to malformations in embryos, which is why valproic acid must not be used in pregnant women.
In addition, however, valproic acid also makes it a possible active ingredient in cancer therapy, since the regulation of gene expression is an essential aspect of tumorigenesis. By modulating gene activity, valproic acid is able to either enable normal gene activity by removing gene blockages, or to induce cell death. This effect of valproic acid is currently being further researched.
Medical application & use
Valproic acid is used as an anti-epileptic. It is indicated for generalized forms of epilepsy, for wake-up grand mal epilepsy and adolescent myoclonic epilepsy, for bipolar disorder, for psychoses from the schizophrenic type, for addictions, for therapy-refractory depression, for migraine prophylaxis and for the prophylaxis of cluster headaches . Valproic acid is not approved for the last two areas of application, although it is effective.
Valproic acid may only be used in small children if other anti-epileptic drugs cannot be used. There is insufficient evidence of the benefit for long-term phase prophylaxis in bipolar disorder, which is why there is no approval for this indication.
Risks & side effects
Valproic acid must not be used in pregnant women as it leads to deformities of the embryo. There is also evidence that the use of valproic acid during pregnancy leads to cognitive impairment in children. Problems of verbal skills and memory are particularly common here. In addition, children often experience disorders ranging from the autism spectrum to actual autism. Valproic acid should also not be used during breastfeeding.
Various side effects can occur during therapy with valproic acid. These often include itching and rashes, headache, dizziness, unsteady movement and visual disturbances, loss of appetite or an increase in appetite, weight loss or increase, drowsiness, tremors (tremor), nystagmus (uncontrolled, rhythmic movement of an organ; usually the eyes), temporary Hair loss, severe and sometimes fatal liver damage, hearing loss, paresthesia and sensory disorders, Parkinson-like movement disorders as well as changes in the blood count and blood clotting disorders.
The ammonium concentration in the blood is often increased. Occasionally there are behavioral disorders, bleeding, gastrointestinal complaints, pleural effusion, indigestion, increased salivation, increased blood insulin concentration, edema, delusions, menstrual disorders, temporary damage to the brain, coma, inflammation of the blood vessels and skin rashes.
Tinnitus, a myelodysplastic syndrome, an underactive thyroid, a chronic encephalopathy with disorders of the brain function, severe skin reactions, lupus erythematosus, an impairment of the bone marrow function, disorders of the kidney function (Fanconi syndrome), hyperacidity (metabolic acidosis), and wetting occur rarely , Disorders in the metabolism of the red blood pigment (porphyria), infertility in men, increased testosterone in the blood (in women) and cystic changes in the ovaries and inflammation of the oral mucosa.
Fever, swelling of the face, mouth and neck, lymphocytosis, biotin deficiency in children, hallucinations, swollen gums and a reduced body temperature are also possible.



















.jpg)



