The red dye Clofazimine is used as a medicine in human medicine to treat a leprosy disease. The active ingredient is suitable for this because of its bactericidal properties. Off-label, d. H. Outside the approval area, there is also an area of application for the treatment of massive diseases of the skin.
What is clofazimin?
Clofazimin is a red dye. Due to its bactericidal properties (bactericidal properties), it is used to treat leprosy. In addition, there is a field of application for the drug that goes beyond approval. This is because it is used outside of the area approved by the licensing authorities authorized by public law (so-called off-label use) in order to treat serious skin diseases.
According to the specified area of application, clofazimin should be used together with other leprosy drugs in order to prevent the development of resistance. These include dapsone and rifampicin.
In chemistry and pharmacology, clofazimine is described with the empirical formula C 27 - H 22 - Cl 2 - N 4. This corresponds to a moral mass of 473.39 g / mol. The literature reports that the chemical properties of clofazimine are very similar to those of the cationic amphiphilic drugs (CAD).
Pharmacological effect on the body and organs
The exact effect of clofazimin on the human body has not yet been fully clarified. Various approaches have been developed in science to explain the bactericidal effects of the red dye. However, all explanatory models have in common that they understand clofazimin as a functional inhibitor of acid sphingomyelinase (English: Functional Inhibitor of Acid SphingeMyelinAse, short: FIASMA). It is therefore considered certain that clofazimin inhibits the enzyme acid sphingomyelinase. The red dye is also indisputably considered to be at least slightly bactericidal.
Apart from the mechanism of action, clofazimin can be considered well researched. The melting point of the substance, which is a brownish powder at room temperature, is approx. 212 degrees Celsius.
Medical application & use for treatment & prevention
Clofazimin is now only approved in France within the European Union. Here the active ingredient is sold under the trade name Lamprene®. In the Federal Republic of Germany this preparation could be imported by pharmacies from abroad until 2005. Since some legislative changes, which u. a. led to the loss of approval, clofazimin is only available from the WHO. In exceptional cases, they can also be obtained directly from the manufacturer.
In France and other approved countries, clofazimine is primarily used to treat leprosy. The active ingredient is prescribed for oral ingestion as a film-coated tablet and is subject to pharmacy and prescription requirements. A purchase is therefore only possible after a doctor's prescription in a state-licensed pharmacy.
Clofazimin is indicated for the treatment of leprosy. In this case, however, the therapy always takes place together with other drugs such as rifampicin or dapsone.
Off-label allocation as a drug for the treatment of massive skin diseases is also possible. These include u. a. granulomatous mycoses and the Melkersson-Rosenthal syndrome.
Risks & side effects
Clofazimin can cause undesirable side effects even if taken completely correctly. However, this also applies to other drugs. It is imperative not to use it if an intolerance or allergy to the active ingredient is known.
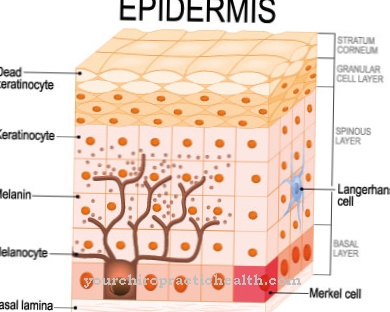
According to the literature, numerous studies have shown that there is a connection between the consumption of clofazimin and the development of skin discoloration. In some test persons, there was an increased development of red or brown-black areas, especially in areas exposed to light. In addition, discoloration of the stool, urine, sputum or hair can occur. Discoloration of sweat has also been reported.
Furthermore, after taking clofazimin, cornification disorders of the skin (in experts: ichthyosis) can occur.
Other side effects are disorders of the gastrointestinal tract, which are primarily expressed in diarrhea, stomach or stomach pain, vomiting and loss of appetite. The development of a pathogenically increased sensitivity to light (photosensitivity) is a potential side effect to be considered.





.jpg)