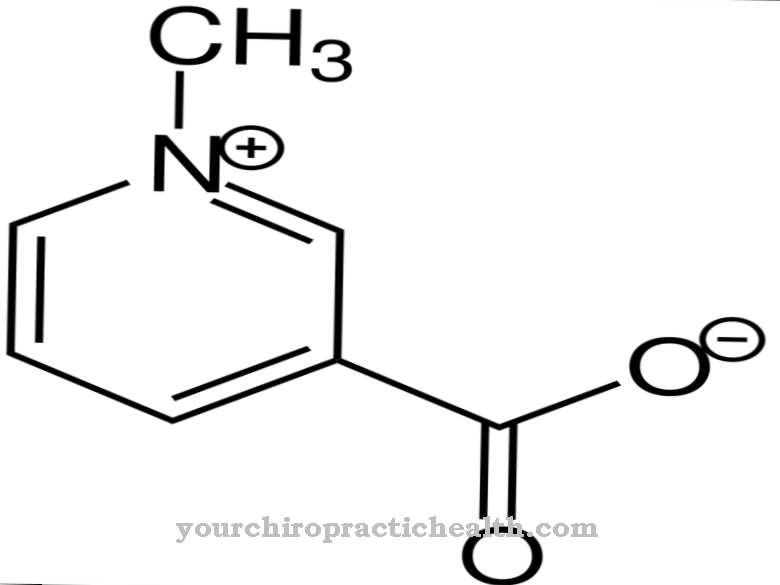
Desmosin is a proteinogenic amino acid. Together with other amino acids, it forms the fiber and structural protein elastin. With mutations in the ELN gene, the structure of the elastin is disturbed.
What is desmosin?
Amino acids are an important part of the human organism. They are a class of organic compounds that are formed from at least one carboxy group and one amino group. Amino acids are therefore both carboxylic acids and amines.
Depending on their position in relation to the carboxy group, amino acids can be assigned to different groups. Amino acids with a terminal carboxy group are called geminal or α and count among the α-amino acids. These amino acids are elements of proteins. The human body has more than 20 proteinogenic amino acids and 400 non-proteinogenic amino acids. The D-amino acids are a special group. One of the more than 20 proteinogenic amino acids is desmosine, which together with the similarly built isodesmosine forms the fiber protein elastin.
The elastin and its soluble precursor tropoelastin belong to the structural proteins and contribute to the shaping and retention of anatomical structures. Elastin plays a special role in the ability of large blood vessels to stretch, for example the aorta.
Function, effect & tasks
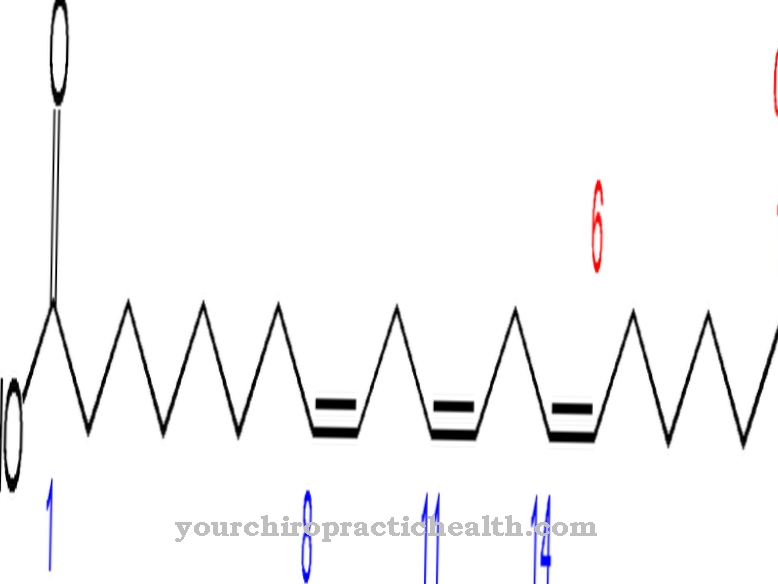
Desmosin is formally a quadruple amino acid. It has a pyridinium ring in the center. Pyridine is a chemical compound with the empirical formula C5H5N, which can be assigned to the heterocyclic parent systems and forms the simplest azine in the form of a six-membered ring with one nitrogen atom and five carbon atoms.
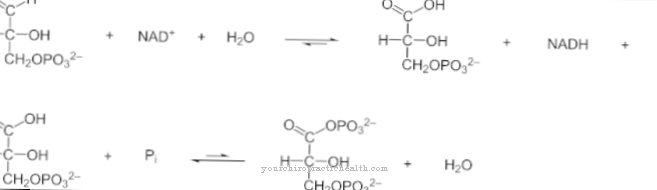
Thanks to its central pyridinium ring, desmosin can network the individual protein strands in the fiber protein elastin. The composition of elastin is similar to that of collagen. Instead of hydroxylysine, however, elastin has a significant proportion of valine. Lysine residues are oxidized to allysine by the enzyme lysyl oxidase. Three allysines and one lysine in turn form a desmosine in the form of a ring. This shape plays a significant role in the elasticity of an entire elastin molecule.
As a protein network, elastin consists of desmosin-linked units and is elastically stretchable. The lungs as well as the skin and blood vessels are dependent on elastin and its component desmosin, as this is the only way they get their considerable elasticity. Desmosin fluoresces blue under UV light and gives elastin its yellow color, its insolubility in water, heat stability and resistance to alkalis and proteases.
Education, occurrence, properties & optimal values
The formation of desmosin is also known as desmosin biosynthesis. During this biosynthesis, the terminal amino groups of L-lysine units are converted into ω-aldehydes by the enzyme lysyl oxidase through oxidation.
Lysyl oxidase is a protein lysine 6 oxidase and thus corresponds to an enzyme that occurs in the extracellular space of the connective tissue. In the cross-linking of elastin and collagen, it serves as a catalyst and mechanical stabilizer for proteins. During the biosynthesis of desmosine, the lysyl oxidase converts lysine into allysine. This process takes place in the extracellular matrix and stabilizes the cross-links between collagen and elastin. From a chemical point of view, the reaction corresponds to oxidative deamination to form the aldehyde. Allysine forms either allysinaldol or desmosine with aldehyde residues of neighboring tropelastin molecules through an aldol condensation.
Remaining lysine forms a Schiff base via its amino group and creates isodesmosine. In addition to the blood vessels, the lungs and the skin, all microfibrils in particular carry desmosin. These are the smallest fibers of collagenous, reticular and elastic tissue.
Diseases & Disorders
The formation of elastin from components such as desmosin is disrupted in various diseases. These diseases primarily include mutations in the ELN gene. The most important of these are dermatochalasis, Williams-Beuren syndrome and subvalvular congenital aortic stenosis. Dermatochalasis is a group of connective tissue changes with familial accumulation.
Characteristic for this group is the sagging, less elastic and wrinkled skin on various parts of the body. The ELN gene codes for elastin and can cause such symptoms through a mutation. Williams-Beuren syndrome is rather rare compared to this, affecting only one in 20,000 newborns. The disease is caused by a defect on chromosome seven. The gene locus is 7q11.23. Due to a defect at this point, the affected person lacks the elastin gene and neighboring genes. The deletion of the elastin gene causes facial dysmorphism and disorders in the internal organ structure. Heart defects such as supravalvular aortic stenosis and kidney malformations such as the horseshoe kidney or renal vascular stenosis can result. In addition, there is often a cognitive disability.
The mental abilities of those affected are below average. Despite verbal expressiveness, they mostly form sentences with little content. They start reading at an extremely early age, which often overestimates their mental abilities. In addition to their hyperlexia, their perfect pitch often leads to overestimations. As a form of elastin mutation, subvalvular congenital aortic stenosis corresponds in turn to a heart malformation associated with a narrowing of the major artery. The supravalvular stenosis lies over the aortic valve at the beginning of the aorta.
This form of the heart defect is often characterized by hourglass-shaped constrictions that lie above the outlet of the coronary vessels. The ascending part of the aorta can also be narrowed. This form of aortic stenosis occurs particularly often in the context of the Williams-Beuren syndrome just discussed. This heart defect has already been observed regardless of the disease. In this case, however, it does not necessarily have to be related to a mutation in the elastin gene.



















.jpg)



