Electrolytes play an important role in many functions in the human body. If the body's own electrolyte balance is impaired, it can cause serious illness.
What are electrolytes?
Electrolytes are chemical compounds and act as a form of so-called ionic conductor. This means that electrolytes allow electrical charges to be transported. Among other things, this works due to the movement of ions (atoms or molecules that are electrically charged).
Electrolytes can be in liquid or solid form: In principle, liquids are always electrolytes when they contain ions, because in liquids, ions usually have the ability to move. But some solids also contain mobile ions and are thus able to serve as electrolytes.
While the ions of some solid electrolytes are already mobile at room temperature, other solids first require high temperatures so that the ions contained can become mobile and the solids can be used as electrolytes.
Meaning & function
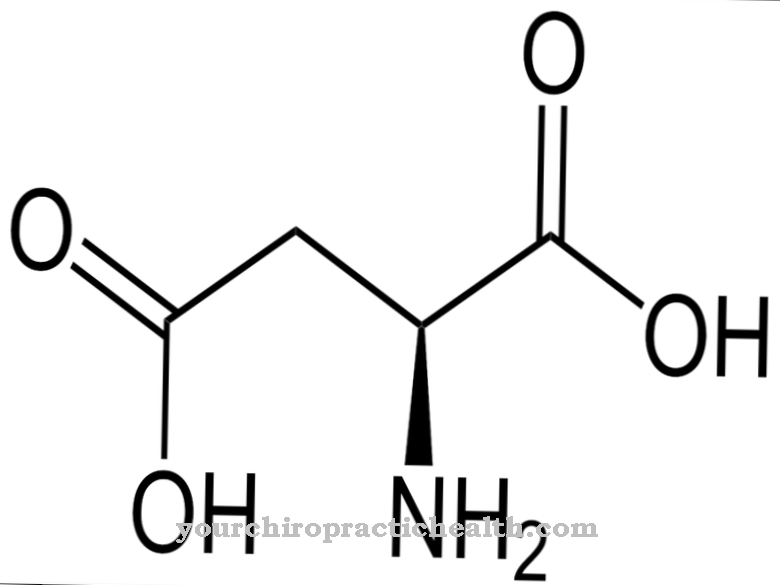
Various Electrolytes, which play an important role for the human body and its health, are also known as biological electrolytes. These biological electrolytes are required for various cell functions, among other things. Corresponding electrolytes are, for example, calcium, potassium, magnesium and sodium.
In the healthy human body, the electrolytes that are inside cells (intracellular electrolytes) and the electrolytes that are outside of the cells (extracellular electrolytes) always maintain a certain balance. This balance of electrolytes is an important prerequisite for regulating the water balance, for example. Various body fluids are affected by the water balance, such as the cerebral fluid, the bile fluid, the synovial fluid and fluids that are present in the stomach and intestines.
In addition, a balance of electrolytes is necessary to regulate the blood pH value: In a healthy body, this value must be within very narrow limits. The lower the blood pH value, the less oxygen the oxygen-transporting proteins in the blood (known as hemoglobin) can bind to.
Electrolytes also play an important role in the functionality and interaction of nerve cells and muscle cells. Within these cells, the concentrations of different electrolytes are regulated, among other things, by ion channels (at these points ions can pass through the cell walls).
A physical balance of different electrolytes is maintained through the absorption of nutrients containing electrolytes. Electrolytes that the body does not need are usually excreted. The absorption and release of appropriate electrolytes is regulated primarily by various endogenous hormones.
Dangers, disorders, risks & diseases
The body's own Electrolyte balance of humans can, among other things, be impaired by an excessive loss of various electrolytes. This can happen, for example, through vomiting, diarrhea or profuse sweating.
In addition, excessive alcohol consumption or malnutrition can lead to a lack of electrolytes. And disorders of the endocrine glands (glands that produce hormones and then release them into the bloodstream) can negatively affect the electrolyte balance.
From one Electrolyte disorder In medicine, one speaks when the measured electrolyte level in a person deviates significantly from a normal level. If such a disturbance of the electrolytes is present for a longer period of time, this can lead, among other things, to impairment of the nervous system and to heart problems. As a result of a disturbance in the electrolytes, for example, the pH value in the blood can drop, which then leads to so-called acidosis (over-acidification). If the blood pH value is increased accordingly, one speaks of an alkalosis.
If the electrolyte disorder is very pronounced, it can in some cases lead to organ failure and even death of an affected person. Serious electrolyte disorders are therefore often treated as medical emergencies. If serious electrolyte imbalances occur, they usually show up in relation to the electrolytes sodium, potassium or calcium. If the electrolyte level is increased during an electrolyte disorder, this is indicated by the prefix 'hyper' (e.g. 'hypernatremia'), if the concentration of certain electrolytes is decreased, this is indicated by the prefix 'hypo' (e.g. 'hyponatremia ').




















.jpg)



