fluorine represents a chemical element with the atomic number 9 and belongs to the halogens. It is a strongly corrosive gas, which causes severe damage to the mucous membranes. Fluorine is used medicinally in the form of its salts, the fluoride, to strengthen teeth.
What is fluorine?
Fluorine is a highly caustic and reactive gas. It is not a compound, but a chemical element that belongs to the halogens. With atomic number 9 it is the lightest halogen. In nature, fluorine occurs mainly in the form of its salts, the fluorides.
The gas fluorine is not very stable and reacts with almost all compounds and elements immediately after its production. Only with the noble gases helium and neon there is no reaction. This extraordinarily strong reactivity can be explained by its very strong affinity for electrons. It always withdraws electrons from its reaction partners and is therefore the strongest oxidizing agent. The name fluor is derived from the Latin "fluores" (river). As calcium fluoride (fluorspar) it serves as a flux for ores.
When fluorspar is added to ores, it lowers their melting point so that they become liquid more quickly. From a conceptual point of view, in medicine there is the term fluorine genitalis for the bloodless discharge of secretion from the female genitalia. However, genital fluorine must not be confused with the element fluorine.
Function, effect & tasks
Fluorine is called an essential trace element. However, the importance of fluorine is controversial. It is known that fluorides have protective properties against teeth. Fluoride can strengthen the teeth and at the same time inhibit certain enzymes of caries bacteria, which cause the breakdown of carbohydrates.
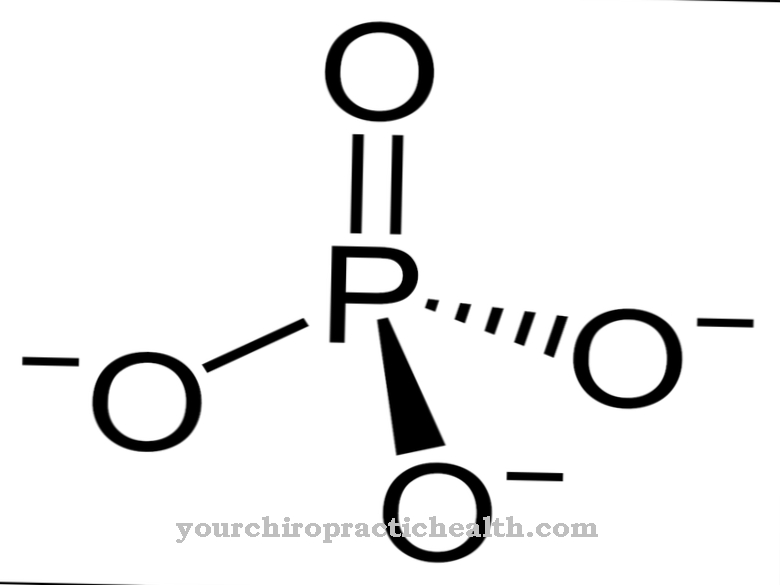
The fluorides act directly on the tooth. Oral ingestion of fluoride has no effect on the teeth. The teeth consist mainly of the mineral hydroxyapatite. Hydroxyapatite can be attacked by acids that are produced by the breakdown of food residues. Poor dental hygiene therefore often results in holes in the teeth that are still occupied by caries bacteria. For example, if the toothpaste contains fluoride, there is an exchange of hydroxyl ions for fluoride ions. This creates fluorapatite, which turns out to be a harder material and less vulnerable to acids. Even hydroxyapatite dissolved by acids can be precipitated again as fluoroapatite in the presence of fluorides.
A beginning destruction can thus be reversed. But fluorides also have positive properties for building bones. Here the intake takes place orally. Children and babies are given fluoride and vitamin D to prevent rickets. However, fluoride should not be overdosed, so that fluorosis with stiffening and thickening of the joints cannot develop. Fluorine compounds are also approved as drugs for osteoporosis. The corresponding tablets contain sodium fluoride or disodium fluorophosphate.
Education, occurrence, properties & optimal values
Fluorine is contained in the form of fluorides in black and green tea, asparagus and also in fish. Many salts contain fluoride. There are no pure fluorine salts due to the low solubility of fluoride-containing compounds in water. Fluorspar (calcium fluoride) and fluorapatite are most common in the earth's crust.
Fluorine is mainly made from calcium fluoride. There are even organisms that can make organofluorine compounds. The South African Gifblaar or plants of the genus Dichapetalum can synthesize fluoroacetic acid against predators. The human organism has a daily requirement of 0.25-0.35 mg.
Diseases & Disorders
However, fluorine-related poisoning and health problems are more common. As mentioned earlier, pure fluorine is a very poisonous corrosive gas. This is also what makes it difficult to make fluorine.
Since it reacts with almost all materials, it can also be stored and transported very poorly. When poisoned with fluorine, chemical burns and burns occur in the lungs, on the skin and in the eyes. Depending on the dose, the relevant organs dissolve within a short time, resulting in death. The lethal dose is very low and is 185 ppm. Fluorine poisoning with pure fluorine will rarely occur because the gas is not stable. However, hydrogen fluoride poisoning is similarly dangerous. Hydrogen fluoride forms hydrogen bonds with the proteins in the body, whereby the tertiary structure of the proteins is destroyed. A denaturation of body protein takes place.
Fluorides can form complex compounds with aluminum ions that have a similar effect to phosphates. In the body, these compounds intervene in the phosphorylation reactions. Among other things, this leads to deregulation of the G proteins, whereby many enzymes are inhibited. For this reason alone, an increased dose of fluoride is not tolerated by the body. Taking too much fluoride tablets can also lead to nausea, vomiting and diarrhea. The fluoride reacts with the stomach acid, whereby a small amount of hydrofluoric acid is formed. This attacks the mucous membranes. A chronic, mild overdose of fluoride can lead to fluorosis.
Fluorosis is chronic fluorine poisoning with changes in the structure of the tooth enamel, coughing, sputum and shortness of breath. Too much hydroxyapatite turns into fluoroapatite in the teeth. The teeth become more brittle. The bones also change due to the excessive formation of fluorapatite. The bones slowly stiffen and remodel. In addition, the enzyme enolase is inhibited.



















.jpg)



