Under the name G proteins is an inhomogeneous group of proteins that can bind the nucleotides guanosine diphosphate (GDP) and guanosine triphosphate (GTP).
They play a crucial role in the transmission and “translation” of extracellular signals into and within the cell. Membrane-bound, heterotrimeric G proteins are the mediator between the extracellular and intracellular space and so-called small G proteins, which are located in the cytosol of the cells, ensure the transmission of the signals within the cell.
What is a G protein?
G proteins, also known as GTPases, represent an inhomogeneous group of proteins that play a crucial role in the transmission of extracellular signals into and within the cell. All G proteins are characterized by the fact that they can bind the nucleotides GTP and GDP.
They can be divided into two large groups of membrane-bound heterotrimeric G proteins and so-called small monomeric G proteins. The monomeric G proteins are located in the cytosol of the cells and act as second messengers for signal transduction within the cell. The membrane-bound G proteins are composed of the subunits Alfa, Beta and Gamma. In the inactive state, GDP is bound to the alpha subunit.
An extracellular stimulus (signal) sets in motion a process in which the GDP is replaced by GTP and at the same time a dissociation takes place between the alpha subunit and the beta-gamma subunit. The two beta and gamma subunits remain together as an active functional unit even in the subsequent processes as a beta-gamma subunit. The exchange of GDP by GTP thus corresponds to switching from the inactive “OFF position” to the activated “ON position”.
Function, effect & tasks
Like animal cells, human cells are protected by a cell membrane that is not easily permeable to large molecules or pathogenic germs. On the one hand, the cell membrane provides protection for the internal cytosol and the cell nucleus; on the other hand, this can be a problem for the necessary communication and information exchange between cells, within a cell and between extracellular and intracellular space.
The main function of the membrane-bound heterotrimeric G-proteins, of which about 21 different alpha subunits are known, consists in signal transduction from the extracellular space to the inside of the cell. Signal transductions are essential for the transmission of signals and the translation of certain “instructions” into cellular metabolic processes. The point is to receive important messages that are brought to the cell from outside via messenger substances, hormones or neurotransmitters and to translate them as "work instructions" for the cell and to pass them on to second messengers inside the cell, which ensure further transport within the cytosol .
The process of transduction also plays an important role in the transmission of certain sensitive stimuli such as sight, hearing, taste and smell. Signal transduction is just as important for the functioning of certain control loops that control body temperature, blood pressure, heart function and many other unconscious parameters. In simple terms, the heterotrimeric G-proteins anchored in the cell membrane embody the active clearing point for signal substances, which are transferred in a transformed form to the small G-proteins inside the cell, which act as second messengers.
The small G proteins, of which more than 100 different forms are known, perform a wide range of tasks within the cell.For example, they are involved in the regulation of gene expression, the organization of the cytoskeleton, the transport of substances between the nucleus and the cytoplasm, as well as the exchange of substances with the lysosomes and cell proliferation.
Education, occurrence, properties & optimal values
As with all other proteins, the basic building blocks of G proteins are the so-called proteinogenic amino acids, 23 of which are known to date. While the cell metabolism is able to synthesize most of the amino acids itself, the few amino acids described as essential must be taken in with food.
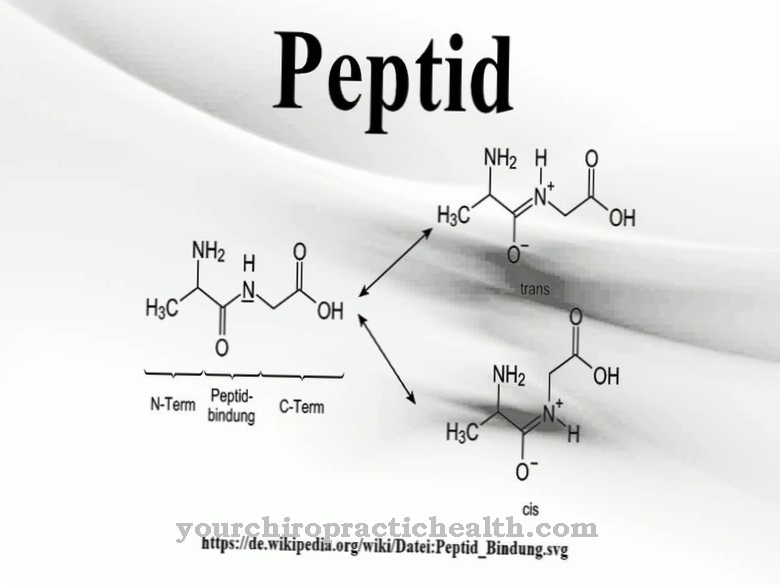
The assembly of the proteins takes place either from the ground up by stringing together amino acids in the genetically specified sequence or by assembling existing fragments of partially dismantled, long-chain proteins. The fragments can also consist of peptides or polypeptides which, according to the definition, are composed of less than 100 amino acids. The synthesis of the G proteins takes place in each individual cell in complex processes based on the gene segments previously copied in the mRNA, which determine the amino acid sequence of each individual protein.
Because G proteins in their diversity are involved in practically all control and regulation processes of every single cell and the relationship between activated and inactivated state is very dynamic, a snapshot of their concentration or activity in the cells is not possible and would not be meaningful. Whether all of the G proteins in the network perform “normal” work can only be assessed indirectly via the health status.
Diseases & Disorders
In the case of proteins that are the functional or activating part of an enzyme, hormone or other functional units, there is a risk that an error in their amino acid sequence will cause them to lose function and the enzyme or hormone to lose part of its effectiveness. In most cases of a “protein defect” there is a corresponding genetic defect.
Mutation of a gene segment leads to an incorrect specification of the amino acid sequence and thus to an incorrect construction of the corresponding protein. The G proteins are not spared from such genetically determined errors in the blueprint. However, the G proteins also lose their function if the fault lies in the G protein-coupled receptors.
In both cases, the reduced ability to transduce signals triggers a certain disease or contributes to its development. Diseases that are associated with impaired function of G proteins are, for example, pseudohypoparathyroidism, acromegaly, hyperfunctional thyroid adenoma, tumors of the ovaries and a few others.