About half of all proteins are in the human body Glycoproteins. The substances play a role as cell components as well as immune substances. They are mainly formed as part of the so-called N-glycosylation and can cause serious diseases if they are incorrectly assembled.
What are glycoproteins?
Glycoproteins are proteins with tree-like branched heteroglycan residues. They are usually of a viscous consistency. The macromolecules contain covalently bound sugar groups.
They consist of monosaccharides such as glucose, fructose, mannose or acetylated amino sugar. That is why they are also known as protein-bound oligosaccharides. The covalent bond can take place in different ways and corresponds either to a bond to the amino acids serine or asparagine. The bond to serine is called O- and that to asparagine N-glycosylation. The glycoproteins involved in N-glycosylation vary in size. They correspond to monosaccharides, di- or oligosaccharides and even polysaccharides.
Depending on their proportion of monosaccharides, they are divided into high-mannose, complex and hybrid glycoproteins. In the mannose-rich group, residues of mannose predominate. In the complex group, saccharides predominate. The hybrid group is a hybrid. The carbohydrate content of glycoproteins is between a few percent for ribonucleases and up to 85 percent for blood group antigens.
Function, effect & tasks
Glycoproteins fulfill numerous functions in the human organism. They are a structural component of cell membranes and are also referred to as structural proteins in this context. They are also found in mucus and are used as lubricants in liquids.
As membrane proteins, they contribute to cell interaction. Some glycoproteins also have hormonal functions, such as the growth factor hCG. The substances are just as important as immunological components in the form of immunoglobulins and interferons. All export proteins and membrane proteins in the body were still glycoproteins, at least during biosynthesis. They are particularly relevant for the recognition reactions in the immune system, as they interact with immunological T cells and T cell receptors. Various plasma proteins have been isolated in human blood plasma, of which only albumin and prealbumin have no sugar residues.
The abundance of glycoproteins is amazing. Ultimately, almost all extracellularly soluble proteins and enzymes have sugar residues. As hormones, glycoproteins have a pleiotropic effect and are therefore crucial for the activity of various organ systems. The hormones TSH, HCG and FSH are for example glycoproteins. As membrane proteins, they are represented in the role of receptor as well as transporter and stabilizer. They have a stabilizing effect, especially together with glycolipids. Together with these substances they form the so-called glycocalyx, which stabilizes cells without cell walls.
Education, occurrence, properties & optimal values
The most common formation of glycoproteins is the N-glycosidic bond or N-glycosylation to asparagine. Sugar binds itself to the nitrogen-free acid amide groups. N-glycosylation takes place in the endoplasmic reticulum. The N-glycosides thus formed are the most relevant glycoprotein group.
In N-glycosylation, the sugar precursor snythetizes on the carrier molecule dolichol independently of the amino acid sequence of the target protein. The OH group at the end of the molecule is linked with diphosphate. An oligosaccharide precursor is formed at the terminal phosphate residue of the molecules. The first seven of the sugars assemble on the cytosolic side. Two N-acetyl-glucosamines and five manose residues are attached to the dolichol phosphate. The sugar nucleotides GDP-mannose and UDP-N-acetyl-glucosamine appear as donors. The precursor is transported through the ER membrane via a transport protein.
The preliminary stage is thus oriented towards the inside of the endoplasmic reticulum, where four mannose residues are added to it. In addition, glucose residues are grown. The 14 sugar long precursor is finally transferred to a protein. Another formation pathway for glycoproteins is the O-glycosidic bond or O-glycosylation to serine, which takes place in the Golgi apparatus of the cells. The sugar is bound to a hydroxyl group of the serine. Glycoprotein values are particularly relevant in relation to plasma proteins, as they play a role in a complete blood count. To list all normal values for glycoproteins individually at this point would go beyond the scope.
Diseases & Disorders
Some genetic diseases have effects on glycosylation. One group of such diseases is CDG. The glycoproteins show abnormal values. Those affected suffer from slowed development, which relates to both physical and mental issues.
Squint can be another symptom of the genetic disorder. A total of around 250 different genes are involved in the formation of glocoproteins. In the case of congenital glycosylation disorders, disorders in the attachment of carbohydrate side chains to proteins are present due to a genetic disposition. In the post-translational modification, proteins receive their full functionality. In this process, when enzymes or proteins that build carbohydrate side chains are abnormally assembled together, CDG is created. N-glycosylation is most commonly affected by disorders. So far, around 30 enzyme defects have been discovered that have an impact on N-glycosylation.
Genetic O-glycosylation disorders are somewhat rarer. They manifest themselves in neuromuscular multi-system diseases such as Walker-Warburg syndrome. Since glycoproteins take on so many functions in the organism, the clinical picture is characterized by a variety of symptoms. All organ systems can be affected by congenital glycosylation disorders. Psychomotor developmental disorders are the main symptom. Neurological abnormalities are just as common. Coagulation disorders or endocrine disorders are also not uncommon.






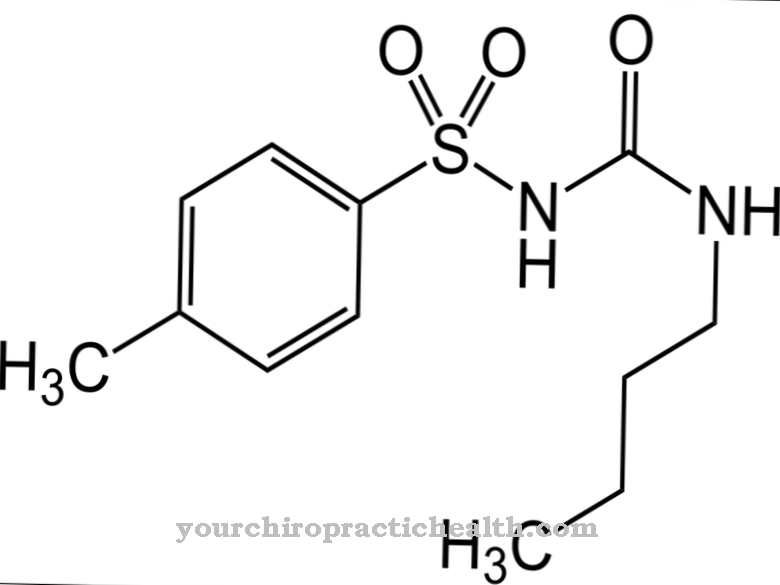

.jpg)
.jpg)
.jpg)



.jpg)