Lysyl oxidase is an enzyme of the connective tissue that has catalytic tasks and promotes the cross-linking of collagen and elastin. The enzyme has a stabilizing effect on the connective tissue by carrying out oxidative deamination and thus creating the basic conditions for cross-linking. In Cutis laxa the activity of lysyl oxidase is reduced.
What is lysyl oxidase?
There are different enzymes in the human body, all of which have catalytic activity. Enzymes enable reactions in the human body or accelerate them. Lysyl oxidase is an enzyme found in human connective tissue. It is also called protein lysine 6 oxidase and is found mainly in the extracellular space of the connective tissue.
The catalytic activity of the enzyme in this case relates to the cross-linking between collagen and elastin. Lysyl oxidase stabilizes the two proteins in a mechanical way and thus enables the reactive connection. Lysyl oxidase is not only found in the human body. Other vertebrates are also equipped with the enzyme. Lysyl oxidase is considered to be a stabilizer of the connective tissue. A deficiency in the enzyme leads to the clinical picture of cutis laxa, a severe and hereditary weakness of the connective tissue.
Function, effect & tasks
Lysyl oxidase takes on important tasks in the extracellular space in the cross-connection between individual collagen molecules. In the human body, collagen plays a major role within the proteins, with about 30 percent of the total protein mass.
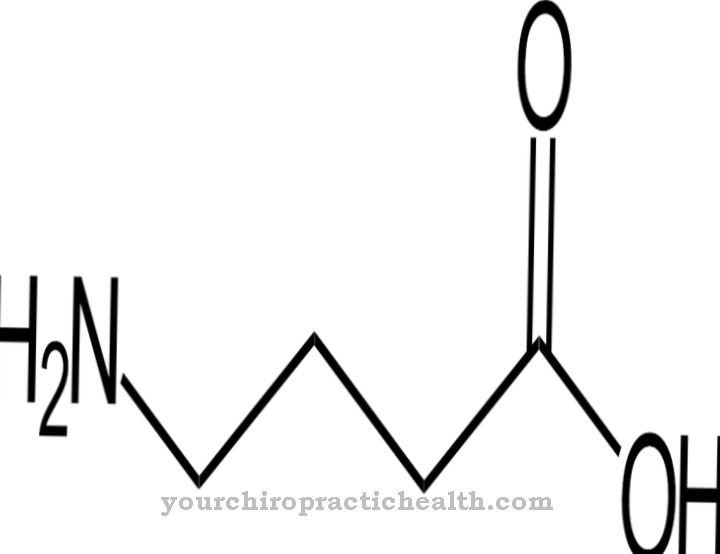
Collagen is the most common protein. It is a structural and building protein that makes up many parts of the body, such as connective tissue, bones, teeth, cartilage, tendons, ligaments and skin. Lysyl oxidase supports the binding of collagen to carbonyl groups and thus contributes to the stability of the body components mentioned. It has catalytic activity for the production of carbonyl groups that form covalent cross-links on collagens in aldol condensations. The catalytic task of lysyl oxidase is therefore to prepare for fibril formation. The enzyme creates all the chemical conditions that are necessary for formation.
Fibrils are considered fibers of fiber. They correspond to thin and fibrous parts of the body and are found in plant cell walls, in human muscles and in connective tissue. The task of lysyl oxidase in this context is essentially the oxidative deamination of lysyl residues. In chemistry, deamination is the chemical splitting off of amino groups as ammonium ions or ammonia. Oxidative deamination splits amino groups of the amino acid L-glutamate from hydrogen and oxidizes them to imino groups with the transfer of hydrogen to NAD + or NADP +.
This is followed by hydrolytic cleavage of imino groups as ammonium ions, which is associated with the formation of α-keto acid. Deamination corresponds to the first step in the biochemical breakdown of amino acids, which in mammals mainly takes place in the liver. The ammonium ion formed during deamination is converted into urea. The deamination processes of the lysyl oxidase give rise to aldehyde groups which, together with the individual amino groups of other lysyl residues, give rise to so-called Schiff's bases and in this way can form the stabilizing cross-links in the collagen.
Education, occurrence, properties & optimal values
Lysyl oxidase in the DNA is encoded by the LOX gene, which in humans is located on chromosome 5 in the gene locus q23.3 to q31.2. The gene product is not the final form of the enzyme. The product is not a finished lysyl oxidase, but a predecessor form which, after translation, has a molar mass of 47 kDa.
Glycosylation occurs in the further course. During this process, the molar mass of the later enzyme increases to 50 kDa and the predecessor form of lysyl oxidase is secreted into the extracellular space. After secretion, the pre-pro-lysyl oxidase is processed further. The substance is split in the extracellular space. Protein 1 is responsible for splitting into two fragments. In this way, on the one hand, the 32 kDa lysyl oxidase is produced. On the other hand, a residual substance is created, which in this case corresponds to a polypeptide.
Diseases & Disorders
Genetic defects in lysyl oxidase can cause the clinical picture of cutix laxe. This disease is also called dermatochalasis and refers to a group of often age-related weaknesses of the connective tissue, which in most cases are observed with familial accumulation.
The common characteristic of all dermatochalasis phenomena is sagging and inelastic skin, which often hangs down in large folds on various parts of the body. Most of those affected look older than they are because of the changes. The diseases are caused, among other things, by genetic mutations. In this context, we are talking about cutis laxa syndrome. The disease can exist in autosomal recessive, autosomal dominant and x-chromsomal forms. In many cases, the cutis laxa syndrome is associated with other anomalies and, for example, can be fatal if the organs are involved.
ARCL1 corresponds to a cutis laxa of autosomal recessive type 1 and is considered to be the most severe form, which under certain circumstances can lead to life-threatening complications. The form ARCL1A is associated with mutations in the FBLN5 gene at locus 14q32.12. Type ARCL1B is associated with mutations in the EFEMP2 gene at locus 11q13.1 and variant ARCL1C corresponds to a cutis laxa with accompanying anomalies in the lung, gastrointestinal and urinary tract, which are due to mutations in the LTBP4 gene at locus 19q13.2.
The mutations in the genes mentioned lead to a below-average activity of lyxyloxidase. Inadequate cross-connections are created due to the reduced activity of the enzyme. The patient's connective tissue is not sufficiently stabilized.




.jpg)

.jpg)


.jpg)