The Transfer RNA is a short-chain RNA, which is composed of 70 to 95 nucleobases and, in two-dimensional view, has a clover-like structure with 3 to 4 loops.
For each of the 20 known proteinogenic amino acids there is at least 1 transfer RNA that can take up "its" amino acid from the cytosol and make it available for the biosynthesis of a protein on a ribosome of the endoplasmic reticulum.
What is Transfer RNA?
The transfer RNA, internationally known as tRNA abbreviated, consists of approx. 75 to 95 nucleobases and, in the two-dimensional plan view, is reminiscent of a cloverleaf-like structure with three unchangeable and one variable loop and the amino acid acceptor stem.
In the three-dimensional tertiary structure, a tRNA molecule is more like an L-shape, with the short leg corresponding to the acceptor stem and the long leg to the anticodon loop. In addition to the four unchanged nucleosides adenosine, uridine, cytidine and guanosine, which also form the basic building blocks of DNA and RNA, part of the tRNA consists of a total of six modified nucleosides that are not part of DNA and RNA. The additional nucleosides are dihydrouridine, inosine, thiouridine, pseudouridine, N4-acetylcytidine and ribothymidine.
In each branch of the tRNA, conjugating nucleobases form with double-stranded sections analogous to DNA. Each tRNA can only take up a certain of 20 known proteinogenic amino acids and transport it to the rough endoplasmic reticulum for biosynthesis and make it available there. Accordingly, at least one specialized transfer RNA must be available for each proteinogenic amino acid. In reality there is more than one tRNA available for certain amino acids.
Function, effect & tasks
The main task of the transfer RNA is to allow a specific proteinogenic amino acid from the cytosol to dock on its amino acid acceptor, to transport it to the endoplasmic reticulum and to attach it there via a peptide bond to the carboxy group of the amino acid that was deposited last, so that the protein that is formed can be attached extended by one amino acid.
The next tRNA is then ready again to store the “correct” amino acid according to the coding. The processes take place at high speed. In eukaryotes, including human cells, the polypeptide chains lengthen by around 2 amino acids per second during protein synthesis. The average error rate is around one amino acid per thousand. This means that roughly every thousandth amino acid was sorted incorrectly during protein synthesis. Obviously, in the course of evolution, this error rate has leveled off as the best compromise between the necessary energy expenditure and possible negative error effects.
The process of protein synthesis takes place in almost all cells during growth and to support the rest of the metabolism. The tRNA can only fulfill its important task and function of selecting and transporting certain amino acids if the mRNA (messenger RNA) have made copies of the corresponding gene segments of the DNA. Each amino acid is basically encoded by the sequence of three nucleic bases, the codon or triplet, so that the four possible nucleic bases are mathematically 4 to the power of 3 equal to 64 possibilities. However, since there are only 20 proteinogenic amino acids, some triplets can be used for control as start or end codons. Also, some amino acids are encoded by several different triplets.
This has the advantage that a certain error tolerance towards point mutations is achieved, either because the incorrect sequence of the codon happens to encode the same amino acid or because an amino acid with similar properties is incorporated into the protein, so that in many cases the protein synthesized is ultimately free of errors or its functionality is just a little limited.
Education, occurrence, properties & optimal values
Transfer RNAs are present in almost all cells in different amounts and different compositions. They are encoded like other proteins. Different genes are responsible for the blueprints of the individual tRNAs. The responsible genes are transcribed in the cell nucleus in the caryoplasm, where the so-called precursors or pre-tRNAs are also synthesized before they are transported through the nuclear membrane into the cytosol.
Only in the cytosol of the cell are the pre-tRNAs, by splicing off so-called introns, base sequences that have no function on the genes and are only carried along, but are still transcribed. After further activation steps, the tRNA is available for the transport of a certain amino acid. The mitochondria play a special role because they have their own RNA, which also contains genes that genetically define tRNAs for their own needs. The mitochondrial tRNAs are synthesized intramitochondrially.
Because of the almost universal participation of different transfer RNAs in protein synthesis and because of their rapid conversions, no optimal concentration values or reference values with upper and lower limits can be given. The availability of corresponding amino acids in the cytosol and other enzymes that are able to activate tRNAs is important for the function of the tRNAs.
Diseases & Disorders
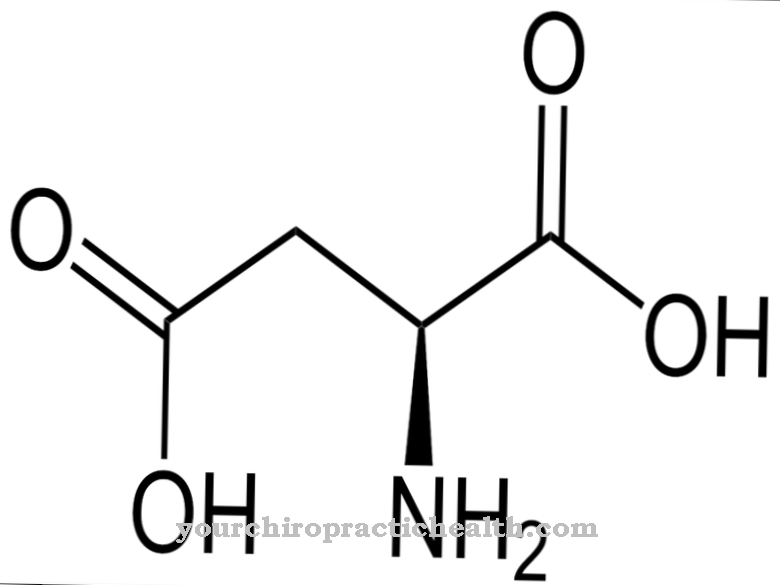
The greatest dangers for a disruption of the function of the transfer RNA lie in a lack of supply of amino acids, especially a lack of essential amino acids, which the body cannot compensate with other amino acids or with other substances.
With regard to real disturbances in the function of the tRNAs, the greatest danger lies in gene mutations, which intervene at certain points in the processing of the transfer RNA and, in the worst case, lead to a functional failure of the corresponding tRNA molecule. Thalassemia, an anemia that is traced back to a gene mutation in intron 1, serves as an example. A gene mutation in the gene that codes for intron 2 also leads to the same symptom. As a result, hemoglobin synthesis is severely restricted in the erythrocytes, so that an inadequate supply of oxygen occurs.





.jpg)

.jpg)


.jpg)