Myofibroblasts are a special form of connective tissue cells. They play an important role in physiological processes, but can also be involved in pathological processes.
What are myofibroblasts?
Myofibroblasts are special cells that represent an intermediate form of connective tissue cells (fibroblasts) and smooth muscle cells. Myo comes from the Greek and is a part of the word meaning muscle. This partial name takes into account the fact that myofibroblasts contain contractile elements that give them properties that are similar to those of smooth muscle cells. They have the ability to have long-lasting contractions (tension) that happen involuntarily.
Fibroblasts are cells that are responsible for building connective tissue when they are active. They produce collagen fibers and molecular components of the basic substance in the extracellular space. Myofibroblasts are able to generate large amounts of collagen if they are stimulated to do so by appropriate factors. They occur in different tissues in which they perform different functions. Accordingly, their formation and differentiation are possible in different ways.
They can arise from embryonic stem cells through direct differentiation, from smooth muscle cells or from certain connective tissue cells in capillary walls (pericytes). Most frequently, however, they arise from not yet fully differentiated fibroblasts in the presence of specific growth factors and signal cells in the tissue.
Anatomy & structure
The cells of myofibroblasts are divided into two parts by their functional structure. The connective tissue part contains a lot of rough endoplasmic reticulum, where a large amount of collagen type III can be produced. This represents a preliminary stage of type I collagen, which is responsible for the build-up and regulated fiber structure in intact connective tissue.
The large Golgi apparatus forms the membranes that are necessary for the construction of the canal system through which the collagen components are transported to their place of action.
The second part of myofibroblast cells has an actin-myosin complex that corresponds to that in smooth muscle cells. Actin and myosin are protein strands that are linked to one another in such a way that they can contract (contract) through an adequate stimulus and with the consumption of energy. In contrast to skeletal muscles, smooth muscle cells are not striated and cannot contract as quickly. But they are able to hold strong tensions for a long time. A special feature of myofibroblasts is the direct connection with the fibronectin threads in the extracellular matrix.
These protein chains form a bridge system with which the cells are networked with one another. Through the connection, the contraction can be transferred to the entire system and thus to larger tissue structures.
Function & tasks
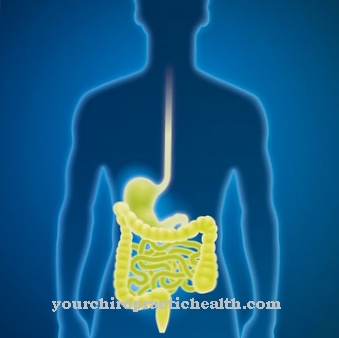
Myofibroblasts are found in the subcutaneous layer of almost all mucous membranes. There they are responsible for maintaining tension and the physiognomy of special types of tissue. The formation of crypts (indentations) and protuberances in the small intestine is largely determined by their contractility.
Maintaining tension and volume in vessels is also one of their tasks, for example in the testicular tubules and capillaries. In contrast to the large arterial blood vessels, these fine tubes do not contain a layer of smooth muscle cells. Due to the presence of myofibroblasts, however, there is a residual function with which the tension of the vessel walls can be adapted to various requirements. Perhaps the most important function of myofibroblasts is to participate in wound healing. The body tries to close tissue defects caused by injuries or other pathological processes as quickly as possible.
The myofibroblasts play an important role in this. The immune defense plays a key role when tissue damage occurs. Among other things, an increasing number of macrophages (scavenger cells) are being sent to the damaged area in order to absorb and phagocytize dead tissue. The appearance of these cells is the initial stimulus for the conversion of fibroblasts into myofibroblasts. These produce large amounts of collagen fibers that are laid like a network over the defective area and form a temporary wound closure. At the same time, they are connected to one another and to the wound edges via the fibronectin threads.
The contraction of all myofibroblasts causes them to be pulled together, an important process for accelerating wound closure. This net-like structure will be rebuilt in further steps. Type III collagen becomes type I, the fibers line up along the direction of pull. The myofibroblasts become inactive and stop their tension activity.
Diseases
The ability of the myofibroblasts to act is fundamentally constitutional and decreases with increasing age. Connective tissue weaknesses are largely determined by these specifications and developments. Regular physical activity cannot stop or reverse this process completely, but it can have a positive long-term effect.
The occurrence of myofibroblasts is dependent on mediators that set their differentiation in motion. If these are missing or only in small numbers, not enough cells are converted. They cannot or insufficiently fulfill the functions that they normally assume. Weaknesses in the immune system in particular can have such consequences, but also genetic defects that affect the growth factors that are important for differentiation.
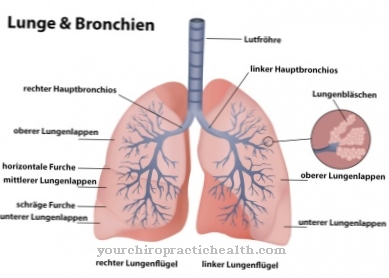
Increased myofibroblast activity can in turn be involved in pathological processes called fibrosis. These are diseases in which the connective tissue structure of organs is strengthened. They are mostly caused by the ingestion of toxins over a long period of time or by autoimmune diseases. As a result, in the course of the disease process, the elasticity of the connective tissue is significantly reduced and the functionality of the affected organs is significantly impaired. Typical examples of diseases caused by toxins are pulmonary fibrosis as a result of increased exposure to coal dust, asbestos or flour dust.
Scleroderma is an autoimmune disease in which the skin and fasciae are affected by the remodeling of the connective tissue. The significant reduction in lung function due to the involvement of the lung fascia is often the reason for the limited lifespan.








.jpg)

.jpg)
.jpg)











.jpg)