A Nucleoside always consists of a nucleobase that is linked to the monosaccharide ribose or deoxyribose via an N-glycosidic bond. All 5 nucleic bases - the building blocks of the DNA and RNA double and single helices - can be enzymatically converted into nucleosides. Some glycosides have a physiological importance such as adenosine, which forms the basic building block for ADP and ATP in the energy metabolism of cells.
What are nucleosides?
The double helices of DNA and the single helices of RNA are formed from sequences of only five different nucleobases in the form of nucleotides.
All five nucleobases, of which adenine and guanine are based on the five- and six-membered ring of the purine and cytosine, thymine and uracil on the aromatic six-membered ring of the pyrimidine, can combine with the monosaccharide ribose or deoxyribose N-glycosidically. The hydroxyl group (-OH) on C atom 1 of the pentose reacts with the amino group (-NH2) of the nucleic base, forming and splitting off a H2O molecule. When a ribose or deoxyribose residue is attached, adenine turns into adenosine or deoxyadenosine.
Similarly, the purine base guanine is also converted into guanosine or deoxyguanosine. The three purine bases thymine, cytosine and uracil are transformed into thymidine, cytidine and uridine by the addition of the ribose residue or are given the prefix “deoxy-” if the sugar residue is made up of deoxyribose. In addition, there is a large number of modified nucleosides, some of which play a role in transfer DNA (tDNA) and in ribosomal RNA (rRNA).
Artificially produced, modified, nucleosides, so-called nucleoside analogs, act e.g. T. as antivirals and are used specifically to combat retroviruses. Some nucleoside analogs have a cytostatic effect, so they are used to fight certain cancer cells.
Function, effect & tasks
One of the most important functions of the five basic nucleosides is to be converted into nucleotides with the addition of a phosphate group to the pentose and to form the building blocks of DNA and RNA as nucleotides.
Some nucleosides also take on tasks in the catalysis of certain metabolic processes in a modified form. For example, the so-called “active methionine” (S-adenosyl methionine) serves as a donor of methyl groups. In some cases, nucleosides also function in their nucleotide form as building blocks of group-transferring coenzymes. Examples of this are riboflavin (vitamin B2), which serves as a precursor for many coenzymes and thus plays a central role in many metabolic processes.
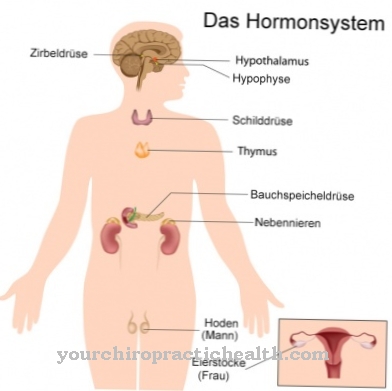
In the energy supply of the cells, adenosine plays a very important role as adenine diphosphate (ADP) and as adenosine triphosphate (ATP). ATP can be described as a universal energy carrier and also serves as a phosphate donor in a large number of metabolic processes that involve phosphorylation. Guanosine triphosphate (GTP) is the energy carrier in the so-called citrate cycle in the mitochondria. Nucleotides are also part of coenzyme A and vitamin B12.
The nucleosides uridine and cytidine are used in combination as drugs for the treatment of nerve inflammation and muscle diseases. For example, the agent is used against nerve root inflammation on the spine and in lumbago. Modified nucleosides, so-called nucleoside analogues, show z. T. virostatic effects against retroviruses. They are used in drugs that are used against z. B. against the herpes simplex virus and against HI viruses. Other nucleoside analogs with cytostatic effects play a role in the fight against cancer.
Education, occurrence, properties & optimal values
Nucleosides are composed exclusively of carbon, hydrogen, oxygen and nitrogen. All substances are abundant practically everywhere on earth. Trace elements and rare minerals are not required for the construction of nucleosides. However, the body does not synthesize nucleosides from scratch because the synthesis is complex and energy consuming.
The human body therefore goes the opposite way; it mainly obtains nucleosides from degradation processes in the intermediate purine and pyrimidine metabolism (salvage pathway). Nucleosides take part in a large number of enzymatic-catalytic metabolic processes in their pure form or in the phosphorylated form as nucleotides. Particularly noteworthy is the function of adenosine in the form of ATP and ADP in the so-called respiratory chain. The nucleotide guanine triphosphate plays a crucial role in the so-called citrate cycle.
During cycles, processes take place within the mitochondria of the cells. Since nucleosides are almost always present in bound form or as functional carriers in practically all body cells in large quantities, there is no general limit or guide value for an optimal concentration. Determining the concentration of certain nucleosides or nucleotides in the blood plasma can be helpful for diagnosis and differential diagnosis.
Diseases & Disorders
Nucleosides are an active part of many metabolic processes and their functions can only rarely be viewed in isolation. Disturbances are usually about complex enzymatic-catalytic processes that are interrupted or inhibited at certain points and lead to corresponding symptoms.
Diseases that cause metabolic abnormalities of the nucleosides mostly also affect the purine or pyrimidine metabolism, because the five basic nucleosides have either a purine or a pyrimidine skeleton. A known disorder in purine metabolism is caused by the well-known Lesch-Nyhan syndrome, an inherited disease that causes a deficiency in hypoxanthine-guanine phosphoribosyltransferase (HGPRT). The lack of enzymes prevents the recycling of certain nucleobases, so that there is a cumulative accumulation of hypoxanthine and guanine.
This in turn triggers hyperuricemia, an elevated uric acid level, which leads to gout. The increased uric acid level leads to deposits on joints and tendon sheaths, which can trigger painful symptoms. A very rare hereditary disease manifests itself in adenylosuccinate lyase deficiency, which leads to problems in purine metabolism. The disease leads to muscle twitching and delayed, serious development of the child.







.jpg)

.jpg)


.jpg)