Phenethylamine (PEA) is the parent substance of catecholamines such as adrenaline, noradrenaline or dopamine. It is often blamed for triggering feelings of happiness. It is found both widely in the plant kingdom and as a hormone in the human body.
What is phenethylamine?
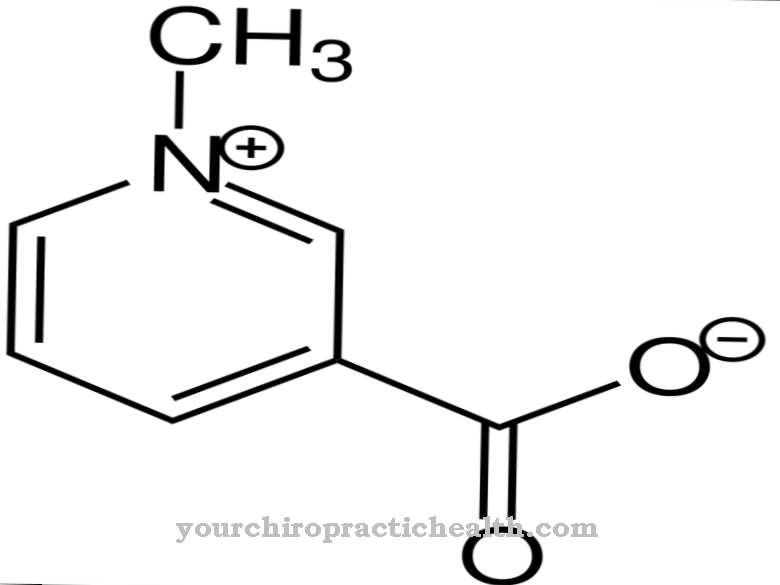
Phenethylamine is considered to be the parent substance for the catecholamines, which are widely used in the body as neurotransmitters or hormones. The active ingredients adrenaline, noradrenaline or dopamine are derived from the parent substance PEA. The correct chemical name for phenethylamine is 2-phenylethylamine.
In the plant, this compound acts as a precursor for the benzylisoquinoline alkaloids. Therefore, this active ingredient is widespread in the plant kingdom. In addition to the important catecholamines that act in the human body, such as dopamine, adrenaline or noradrenaline, many psychedelic hallucinogens are also derived from phenethylamine. Phenethylamine was recognized as the body's own hormone, which is responsible for the creation of feelings of pleasure and happiness.
As a chemical molecule, it consists of an aromatic phenyl ring with a side chain of ethylamine. Phenethylamine is a colorless liquid with a fishy odor and a boiling point of 200 degrees. The compound is poorly soluble in water. It is particularly common in bitter almond oil and cocoa beans. It was also found in the brain and urine.
Function, effect & tasks
Phenethylamine is an endogenous hormone that creates feelings of pleasure and happiness. In the state of greatest happiness, increased concentrations of PEA are found in the body.
The starting point for the biosynthesis of phenethylamine is the amino acid phenylalanine. It has been found that PEA can be distributed both physically and psychologically. For example, studies have shown that exercise increases the levels of phenethylamine in the body. After endurance training, runners get into a state of intoxication, which is due to the high phenethylamine concentrations. Feelings of happiness are also triggered when falling in love.
It was found that people in love also have a higher PEA concentration in their bodies. Here, too, the body is put into a state of intoxication, which causes the famous tingling sensation in the stomach. At the same time, however, rational thinking is restricted, which leads to a certain carelessness or even "blindness". However, the effects of phenethylamine don't last forever. After a period of four years you get used to the increased values. Thereafter, withdrawal symptoms can occur, which lead to a depressed mood. The effect of PEA is similar to that of a drug and the biochemical processes are also similar.
According to some statements, the oral intake of PEA should not have any effect because the active ingredient is broken down very quickly by monoamine oxidase (MAO). Other authors speak of short-term effects that manifest themselves as an increase in blood pressure. The occurrence of a sudden migraine when consuming foods containing phenethylamine is partly explained by the increase in blood pressure.
PEA can bind carbon dioxide. The increased carbon dioxide concentration in the blood not only increases blood pressure, but also increases blood sugar levels and stimulates breathing. If the phenethylamine values are very high, toxic effects on the circulation can also occur. However, the effect is individually different.
Education, occurrence, properties & optimal values
As already mentioned, phenethylamine is very widespread in the plant kingdom, where it serves as a precursor for certain alkaloids. A lot of phenethylamine was found mainly in bitter almond oil or cocoa. The lucky effect of chocolate is said to be due to PEA. At least an increased concentration of dopamine could be determined, which can be formed from phenethylamine.
However, it remains to be seen whether this effect is due to the consumption of chocolate. PEA is broken down very quickly when ingested. The basic chemical structure of the catecholamines, including PEA, allows this group of active substances to appear as neurotransmitters, which characterize them as psychotropic substances. However, the traces of PEA found in the brain or urine are unlikely to come from food. The body itself produces phenethylamine from phenylalanine.
Diseases & Disorders
Elevated concentrations of phenethylamine can be toxic. This enables increased stimulation of the circulatory system, which can lead to cardiovascular problems. In addition, increased phenethylamine concentrations are responsible for the development of migraines.
It has also been observed that greatly increased phenethylamine concentrations in the blood can result in delayed histamine catabolism. In the process, histamine accumulates in the body. The increased histamine concentration has a toxic effect. Difficulty breathing, red skin, hives, nausea, vomiting, headache and diarrhea occur, among other things. The symptoms are reminiscent of fish poisoning. The high phenethylamine concentration responsible for the delayed breakdown of histamine can usually not be generated by an increased intake of phenethylamine because it is quickly broken down by monoamine oxidase (MAO) and would only be increased for a short time.
However, MAOIs limit the action of the enzyme, which increases the phenethylamine concentration. Therapies that contain MAO inhibitors can also lead to severe side effects if used improperly. Phenethylamine has an antidepressant effect. However, PEA is not suitable for the treatment of depression because of its rapid degradability by monoamine oxidase.
However, the administration of monoamine oxidase inhibitors increases the body's own concentration of PEA. So MAOIs can be used to treat depression. Additional PEA intake is contraindicated during this treatment. The lack of degradation of phenethylamine would increase its concentration and possibly lead to significantly increased concentrations. This would lead to an increase in blood pressure, headaches and possibly histamine poisoning.







.jpg)

.jpg)
.jpg)











.jpg)