Phenylalanine is a proteinogenic, essential amino acid with an aromatic six-membered ring that acts as a building block for many proteins and peptides.
Phenylalanine also plays an important role in nitrogen metabolism and can be converted in the liver into the proteinogenic amino acid tyrosine. Phenylalanine and tyrosine play an important role in the synthesis of insulin, melanin, thyroxine and neurotransmitters dopamine, serotonin and tyramine.
What is phenylalanine?
Phenylalanine is an essential alpha-amino acid which - unlike most proteinogenic amino acids - is not only bioactive in the L-form, but to a limited extent also as an enantiomer in the R-form.
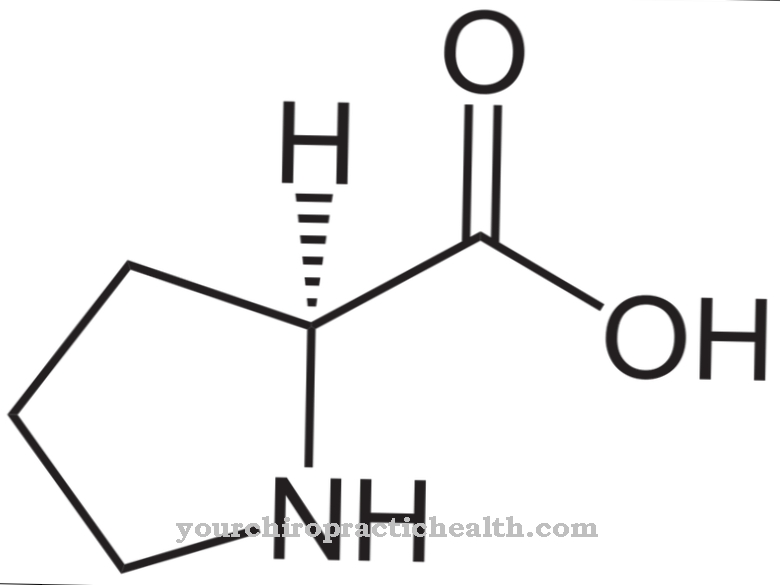
R-phenylalanine is biochemically largely inactive and occurs exclusively in the artificial production of the amino acid, but the role of D-phenylalanine in the control of certain neurotransmitters within the pain complex is being discussed. As a characteristic structural feature, phenylalanine has a simple aromatic six-membered ring (benzene ring) with an attached hydrocarbon chain. The chemical structural formula is C6H5-CH2-CH (NH2) -COOH, with the C6H5 group indicating the benzene ring. The amino acid is amphiphilic, which means that it is both fat and water soluble.
The chemical formula also shows that phenylalanine consists exclusively of carbon, hydrogen, nitrogen and oxygen, substances that are ubiquitous. Rare metals, minerals or trace elements are not part of the amino acid. Nevertheless, the human metabolism cannot synthesize phenylalanine to a sufficient degree from tyrosine, but is dependent on intake from food. Phenylalanine is present in sufficient quantities in many animal and vegetable foods, so that with a normal, mixed diet - provided that the digestive tract is normally absorbed - there is no need to fear a deficiency in the amino acid.
Function, effect & tasks
The most important function and task of phenylalanine is to participate in the structure of many proteins and peptides. It is also involved in the synthesis of some hormones that play a central role in the control of metabolic processes.
They are hormones like adrenaline, noradrenaline, L-dopa, PEA and melanin. In addition, L-phenylalanine serves as the basic substance from which z. B. the messenger substance dopamine, serotonin, tyramine and others can be synthesized. L-phenylalanine also serves as a starting material for the essential amino acid tyrosine. For this purpose, phenylalanine is converted into tyrosine in the liver in two steps by hydroxylation and splitting off a water molecule. Phenylalanine hydroxylase is the enzyme that catalyzes the conversion to tyrosine.
An alternative supply of the also essential amino acid tyrosine can - as with phenylalanine - take place through food intake. In contrast to all other amino acids, which only have a bioactive effect in their L-form, the D-enantiomer of phenylalanine seems to have at least an influence on the perception of pain. A mixture of L- and D-phenylalanine (racemic mixture) was found to have an analgesic effect. The DL mixture is likely to block the breakdown of enkephalins - the body's own opioids - so that the analgesic effect is prolonged and intensified.
Education, occurrence, properties & optimal values
The essential amino acid phenylalanine is absorbed through food. It is not free, but usually as part of a protein or polypeptide in chemically bound form. In order to make the amino acid available for metabolism, the corresponding protein must first be broken down in the course of digestion and then extracted from the "fragments" using further enzymes in the further metabolism.
L-phenylalanine is synthesized via the so-called shikimic acid route. It is a complex biocatalytic chain reaction that autotrophic plants and bacteria have. The special feature of autotrophic organisms is their ability to form organic matter from exclusively inorganic material. Free L-phenylalanine tastes bitter, while its D-enantiomer, which is produced exclusively in industrial production, has a sweet taste. The amino acid is z. B. offered as a dietary supplement and is also part of the artificial sweetener aspartame. Bioavailable L-phenylalanine is found in bound form in many foods.
Their content is particularly high in dried peas and soybeans, in walnuts and pumpkin seeds as well as in various types of fish and meat. The phenylalanine requirement is heavily dependent on the supply of tyrosine. If there is no tyrosine in the diet, the body needs 38 to 52 mg per kg of body mass. With a rich supply of tyrosine in the diet, the daily requirement drops to only 9 mg per kg of body mass. As a rule, foods containing phenylalanine also contain a corresponding amount of tyrosine.
The recommendation of the FAO / WHO from 1985 amounts to a combined requirement for L-phenylalanine and L-tyrosine of 14 mg per kg body mass per day. An adult with a body mass of 80 kg therefore needs 1,120 mg of both substances per day.
Diseases & Disorders
Symptoms of deficiency when there is a permanently insufficient supply of phenylalanine and tyrosine in the diet are extremely rare, but can have serious consequences, especially in the neuronal area.
Apart from an impairment of the synthesis of many hormones and neurotransmitters, the deficiency can also be shown by a disruption in the myelination of nerve fibers. The opposite of a deficiency, an overconcentration of phenylalanine (phenylketonuria), can occur due to a genetic metabolic disorder. The disease is inherited in an autosomal recessive manner and leads to a reduced production of the enzyme phenylalanine hydroxylase, which can convert phenylalanine into tyrosine.
The reduced enzyme activity leads to a sharp increase in the amino acid, to what is known as phenylketonuria, because the conversion to tyrosine is also the breakdown pathway for phenylalanine. At the same time, there is a lack of tyrosine because the synthesis path is blocked. Another hereditary disease in this context is Hartnup syndrome. It is a metabolic disorder that disrupts the transport of phenyalanine across the cell membrane. This leads to serious problems in the CNS, on the skin and in the digestive tract.