As Prophage the phage DNA of temperate bacteriophages is called when it is present in the bacterial host cell. Bacteriophages were discovered by Félix Hubert d’Hérelle in 1917. They are viruses that have adapted to specific bacteria. In the further course of the research, a distinction was made between lytic phage with high virulence and temperate phage with silent prophage and lysogenic cycle.
What are prophages?
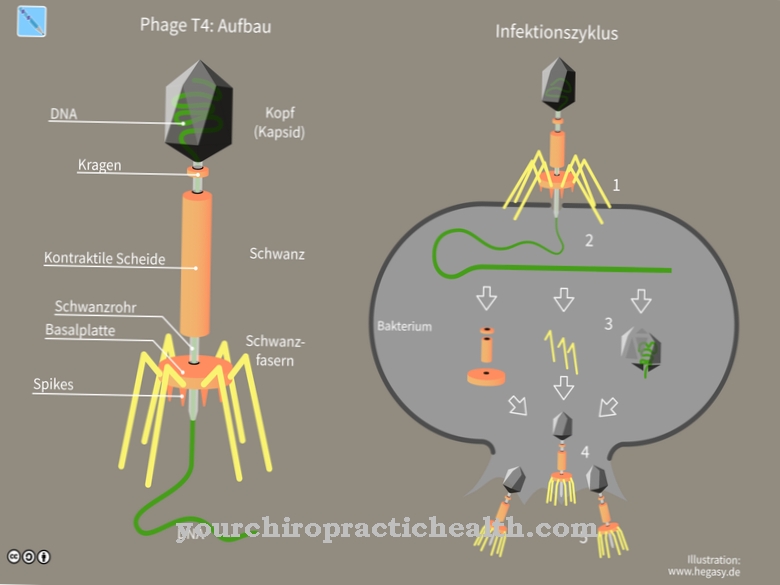
The prophage of temperate bacteriophage can be present as a plasmid in the host cell or be integrated into the bacterial DNA. For this, the temperate phage must adopt the lysogenic cycle when the phage DNA is injected. A distinction is made between the lytic cycle and the lysogenic cycle. While the lytic cycle causes rapid replication and subsequent lysis of the host cell after the injection of the genetic material, in the lysogenic cycle repressor genes from the phage are injected into the host cell in order to suppress the lytic cycle, i.e. the rapid dissolution of the cell.
The temperate phage can switch between lytic and lysogenic cycle depending on the prevailing environmental conditions. The lytic cycle refers to the conventional way the phage genes operate within the host cell. Rapid replication within the host cell takes place after the injection of the viral DNA. After the capsid and the tail fiber proteins have replicated in addition to the viral DNA and numerous new viral particles have been assembled from the individual parts, the cell wall of the host cell is decomposed by lysozyme. When the cell wall dissolves, the new phages are released and their DNA can now be injected into other bacterial cells. This process is completed in about an hour.
Because of the high number of new viral particles, this approach is referred to as the "virulent form". Since the cell wall of the host is destroyed by means of lysozyme, the term "lytic cycle" is used. In the case of temperate phage, the rapid replication and subsequent lysis of the host cell does not necessarily have to come into effect. Depending on the existing environmental factors, the temperate phage can switch between lytic and lysogenic cycle. The lytic cycle can be suppressed by the injection of repressor genes and the lysogenic cycle can start indefinitely.
In the lysogenic cycle, the phage genetic material is inserted into the germ's genetic material and can survive here for an indefinite period of time. The injected genetic material is called "silent" and is defined as "prophage". The prophage can lie as a plasmid in the cytoplasm of the host cell or be integrated into the genetic material of the bacterium.
The integration of the viral genetic material requires a high degree of specialization. The genome of the temperate phages can only be attached to certain positions in the bacterial DNA. Conversely, the genetic material of individual temperate phage strains can always be identified at the same locations in the bacterial genome.
The successful adaptation makes prophages beneficiaries of bacterial cell division. When the host cell divides mitosis, the viral genetic material is passed on. Further transmission to other bacteria can take place through conjugation. Prophages can therefore spread through different transmission routes over entire bacterial strains. Due to environmental influences such as UV light or certain chemicals, the Prophage can switch back to the lytic cycle and strive for aggressive replication.
The prophage also makes use of the host cell's transcription processes: the injected repressor genes of the phage are recognized as DNA damage by certain enzymes of the bacterium and are broken down. The degradation of the repressor genes is self-destructive within the host cell. The lytic cycle can no longer be suppressed and the prophage changes from the lysogenic state to aggressive replication, which ends with the subsequent dissolution of the bacterial cell wall.
Occurrence, Distribution & Properties
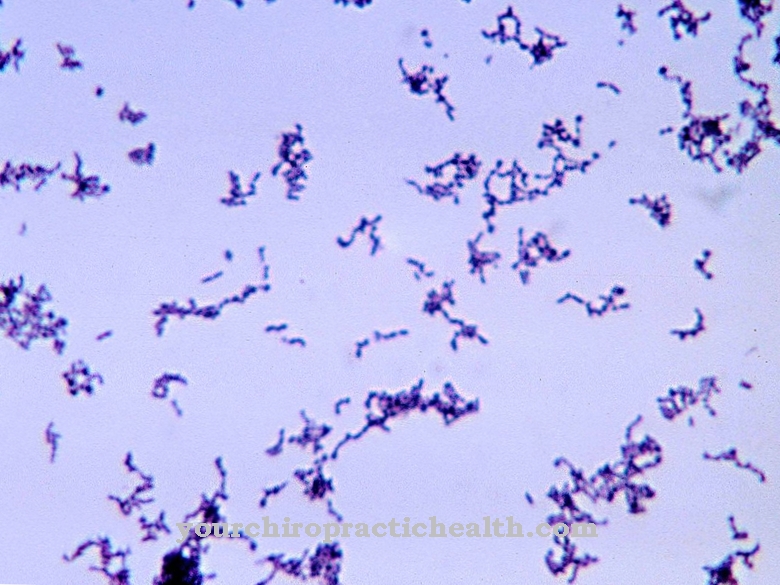
Phages are highly specialized viruses that have adapted to individual strains of bacteria. So not every bacteriophage can access every bacterium. Multiplication without the specific host cell is not possible for the bacteriophage. The high degree of specialization means that bacteriophages can be found in the same terrain as their host cells.
The same is true to an even greater degree for prophages. Since prophages are not conventional viruses and only present themselves as viral hereditary material within the host organism, they cannot be found outside the assigned cells due to the definition alone.
In addition, it must be mentioned that bacteriophages alone in seawater have a number of (10 to the power of 30) and thus more phages than living beings are present on the entire planet. In contrast, there is a very small number of nineteen officially researched bacteriophages, which makes it difficult to make a precise statement about the occurrence.
Meaning & function
Phage therapy was developed in the twenties of the 20th century and is still used successfully in Eastern Europe to combat various infectious diseases. The advantages of phage therapy are obvious: bacteriophages only damage individual bacterial strains, while antibiotics have a generally harmful effect on bacteria in the body.
The discovery of penicillin in the 1940s led to the massive use of antibiotics in the West and, as a result, an end to phage research. The subsequent build-up of numerous antibiotic resistances triggered an increased interest in bacteriophages in the 1990s.
Phage therapy focuses on bacteriophages with aggressive virulence and an exclusively lytic cycle, while temperate bacteriophages and prophages have only played a subordinate role to date.
Illnesses & ailments
Some pathogens can only build up their virulence through symbiosis with prophages. Clostridium botulinum can only produce the dreaded botulinum toxin with the help of the integrated phage DNA. Streptococcus pyogenes can only trigger scarlet fever in combination with prophage DNA.
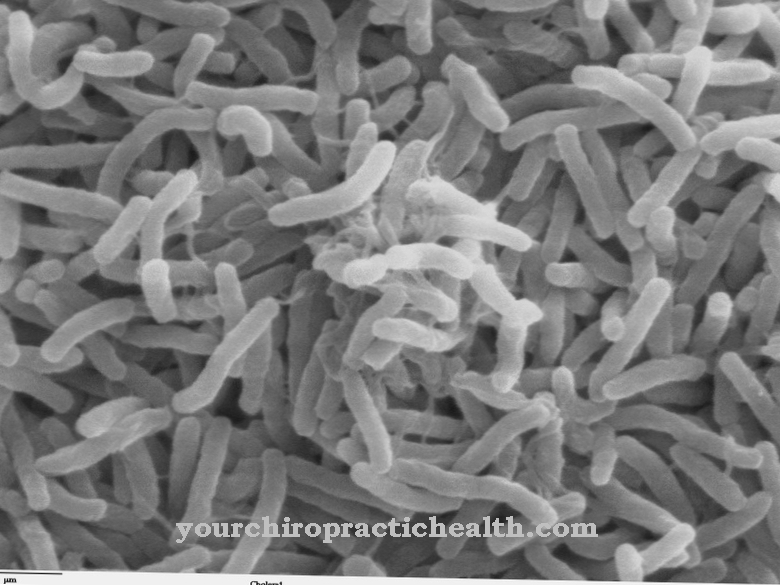
Vibrio cholerae produces cholera only through special prophages. This also shows the importance of phages for human medicine. Entire bacterial strains could lose their pathogenic potential if the responsible prophages could be specifically switched off.







.jpg)


.jpg)



.jpg)
