The biocatalyst Sulfite oxidase causes the conversion of toxic sulfur compounds from the breakdown of amino acids into non-toxic sulfates.
It is vital and therefore occurs in all organisms. If its function is disturbed by a genetic defect, sulfite oxidase deficiency occurs. An excessively high sulfite content in the blood can also have negative effects in otherwise healthy patients.
What is sulfite oxidase?
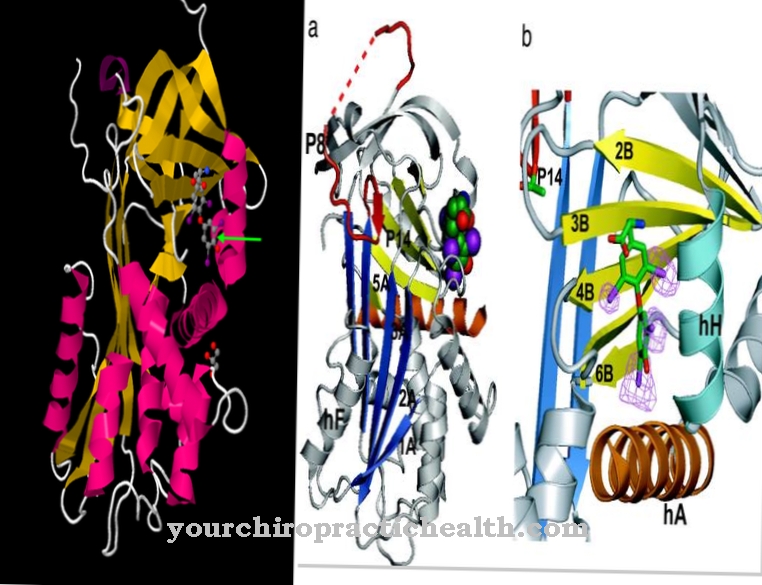
Sulphite oxidase (gene name: SUOX) is the name of a molybdenum-containing enzyme that consists of 466 amino acids. It belongs to the family of xanthine dehydrogenases and occurs in almost all organisms. It contains molybdenum, a vital trace element, in its center.
The metal occurs there in its bioavailable form as a molybdate anion. The sulfite oxidase uses it as a cofactor (molybdate-molybdopterin compound). The enzyme converts the sulfur-containing amino acids methionine, cysteine, etc., which are ingested through food, into harmless sulfur salts (sulfates), which are then excreted in the urine. In mammals, the sulfur-degrading biocatalyst occurs primarily in the liver and kidneys. The enzyme sulfite oxidase ensures that the blood oxygen combines with the essential amino acids and other sulfur substances.
The electrons released are used via the electron transport chain to produce ATP (adenosine triphosphate). The enzyme catalyzes 10 times the amount of sulfites found in one liter of alcohol every day.
Function, effect & tasks
Everyone consumes sulfur-containing proteins and food additives on a daily basis. The latter are contained in pickled vegetables, grapefruit juice, etc. and are intended to protect the food from microbe infestation and discoloration. They form bouquet substances in wine.
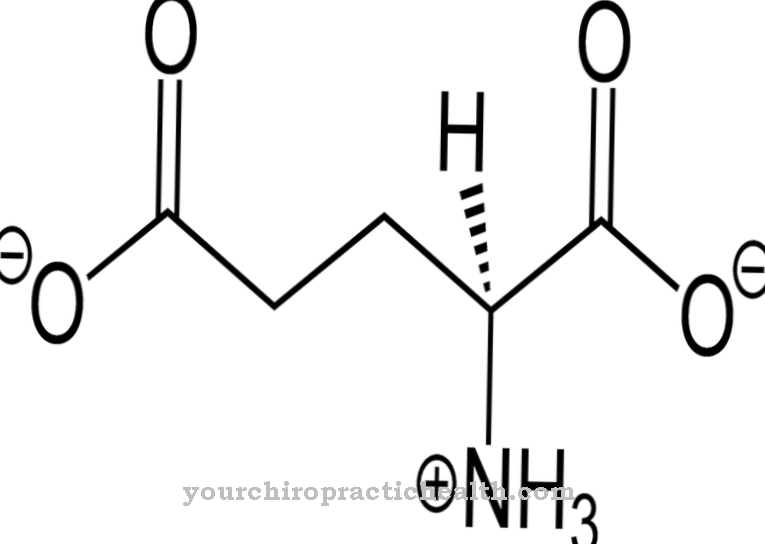
Through the breakdown of cysteine alone, 1680 mg of toxic sulfite are produced in the body every day, which must be converted immediately by the sulfite oxidase so that organs and tissues are not damaged. The enzyme works together with other biocatalysts. Sulphites are poisonous and can destroy vital substances in the body and inhibit necessary metabolic processes even in the smallest quantities. In order to be able to carry out the important detoxification of the cells, the sulfite oxidase needs the trace element molybdenum.
A shortage of this metal can lead to serious consequences. Too high a level of mercury in the body can also inhibit the functionality of sulfite oxidase.
Education, occurrence, properties & optimal values
Sulphite oxidase is mainly produced in the mitochondria, the "energy centers" of the cells. In rats, for example, 80 percent of it occurs in the liver cell mitochondria. In addition, it is still strongly represented in the cells of the kidneys.
The molybdenum oxide required for the activity of sulfite oxidase is located in the active center of the enzyme. As scientists recently discovered, molybdenum trioxide nanoparticles can replace it in patients with a molybdenum deficiency. They have a similar catalytic effect in the body as the natural enzyme. In this way, previously fatal diseases such as sulfocysteinuria could be treated.
Diseases & Disorders
A deficiency in sulfite oxidase can cause asthmatic and even anaphylactic reactions in otherwise healthy people, as the parasympathetic nervous system influences the mast cells responsible for the development of allergies.
In addition, if the sulfite oxidase level is too low, it can lead to severe tiredness, headaches and low blood sugar levels. The genetic deficiency in the vital enzyme has even worse consequences. The newborn is born with physical deformities and mental disabilities. This so-called sulfite oxidase deficiency or sulfocysteinuria occurs as a molybdenum cofactor (MoCo) deficiency disease in an estimated 100,000 to 500,000 births. Infants suffering from an isolated sulfite oxidase defect show similar symptoms: severe encephalopathy, barely controllable seizures, spasticity, microcephaly, muscle relaxation, and progressive brain atrophy.
Since the autosomal recessive inherited enzyme deficiency disease cannot currently be treated effectively, the small patients usually die in childhood: the sulfurous compounds that are not broken down poison neurons and myelin sheaths of the central nervous system and accumulate in cell tissue. Already after birth there are problems with eating and vomiting of the stomach contents. The infants are born with a deformed skull (protruding forehead, deep-set eyes, excessively long eyelids, thick lips, small nose). During the first months of life, the lens shifts in the eye.
About 75 percent of the cases of sulfocysteinuria described so far are caused by a MoCo deficiency: All three enzymes involved in the breakdown of sulfur in the body, sulfite oxidase, xanthine oxidase and aldehyde oxidase, show a greatly reduced activity. The reason for the isolated sulfite oxidase deficiency is a mutation in the SUOX gene (chromosome 12). It appears in three variants: type A (mutation in the MOCS1 gene), type B (MOCS2 gene) and type C (MOCS3 gene). The type A mutation is the most common. The formation of the precursor molecule cPMP is inhibited. However, the substance can now be manufactured and administered in the laboratory.In order to improve the survival of the child patient, the deficiency disease should be diagnosed as soon as possible and treated with daily intravenous administrations of molybdate.
In this way, at least further damage can be contained. The child is given antispasmodic medication to combat the seizures. They must also be on a low-protein diet. Alternatively, MoCo precursor Z can also be administered. It reduces seizures and prevents further brain damage. Medicine has great hopes for the treatment of the previously incurable disease with molybdenum trioxide nanoparticles, which take on the role of sulfite oxidase in the body. To find out whether the unborn child has a sulfite oxidase deficiency, the pregnant woman can have her S-sulfocysteine level in the amniotic fluid checked.








.jpg)

.jpg)
.jpg)











.jpg)