Trypsin As an enzyme of the pancreas, it is responsible for the further breakdown of food proteins. It works in the strongly alkaline range. Trypsin deficiency leads to protein deficiency in the body due to the impaired protein digestion.
What is trypsin?
Trypsin is a protease that continues the breakdown of proteins in the alkaline area of the small intestine. In the stomach, protein breakdown begins in the acidic environment by the enzyme pepsin. The enzyme trypsin consists of three components.
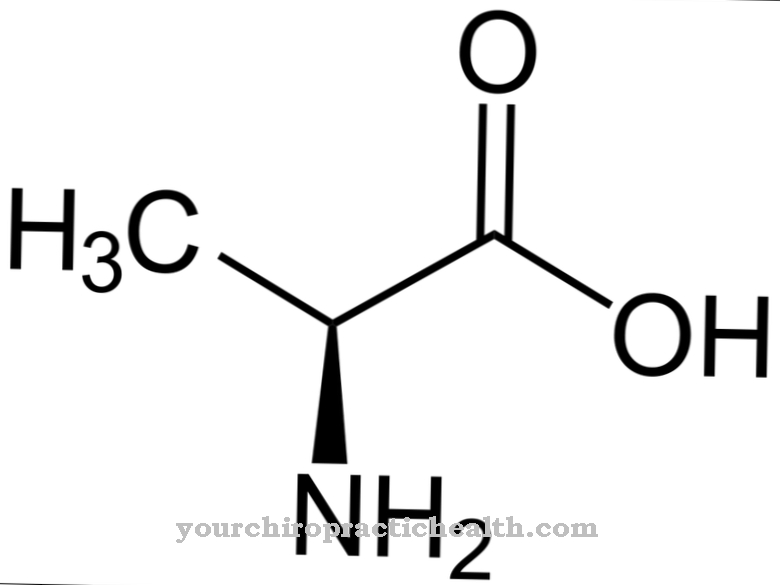
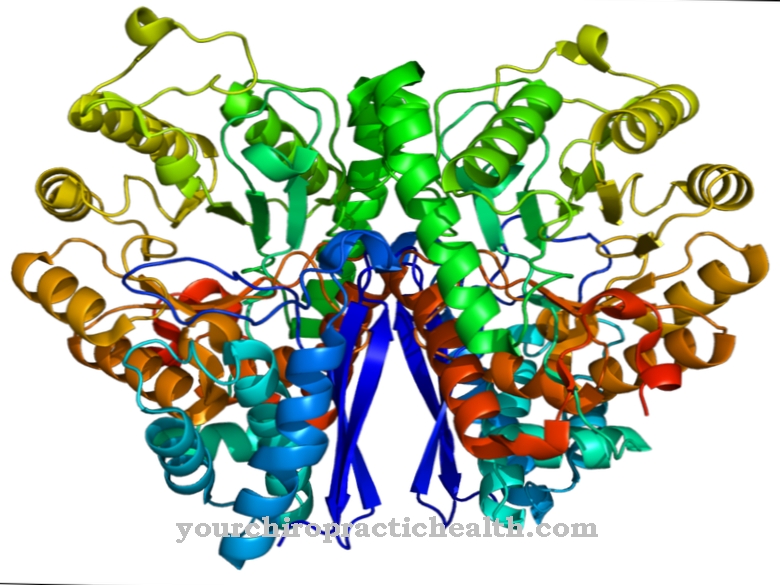
These are trypsin-1 as a cation, trypsin-2 as an anion and trypsin-4. Two thirds of the enzyme consists of trypsin-1 and one third of trypsin-2. Trypsin-4 or mesotrypsin is only found in small amounts. Trypsin is an endoprotease. It only splits a protein in certain places. It is also a serine protease. Its active center contains the catalytic triad of aspartic acid, histidine and serine. It splits the dietary proteins preferentially at the basic amino acids lysine, arginine and modified cysteine.
Trypsin is produced from the zymogen precursor trypsinogen with the help of the catalytic action of the intestinal enzyme enteropeptidase. The enzyme consists of 224 amino acids. Trypsin develops its optimal effect at a pH value of 7 to 8.
Function, effect & tasks
The task of trypsin is to continue the breakdown of proteins that has already started in the stomach in the alkaline range. In the stomach, the pre-digestion of food proteins by the similar enzyme pepsin begins in the acidic range.
Here, too, the protein chains are broken at certain points. While this splitting of the proteins takes place in the stomach on aromatic amino acids such as phenylalanine, the proteins and polypeptides are split by trypsin on the basic amino acids lysine and arginine as well as on the modified cysteine. Another difference to pepsin is that trypsin develops its optimal effect in the alkaline range at a pH value of 7 to 8. Activated trypsin also converts other zymogens such as chymotrypsinogen, pro-elastase, procarboxypeptidase and other inactive enzymes into active enzymes.
The conversion begins immediately after the release of trypsin. The other proteases of the pancreas are chymotrypsin, carboxypeptidase or elastin. Furthermore, trypsin activates itself by converting trypsinogen. The pancreatic enzymes are initially in their inactive form so as not to break down the pancreas through self-digestion. Only when the inactive preforms are secreted can they be activated by splitting. First, the conversion of trypsinogen into trypsin is catalyzed by enteropeptidase. That is the only function of enteropeptidase.
A hexamer with the terminal amino acid lysine is split off from the trypsinogen. Since trypsin also splits off polypeptide chains on the basic lysine, it now also catalyzes its own activation and at the same time the activation of the other zymogens. Together with the enzymes chymotrypsin and elastase, it splits larger proteins in the small intestine and the peptones (polypeptide chains) produced by the action of pepsin into tri- and dipeptides. These smaller peptides are then broken down further into amino acids with the help of other enzymes. In particular, trypsin also contributes to the breakdown of the amino acid methionine. Lysine stimulates, among other things, the formation of trypsin.
Education, occurrence, properties & optimal values
Trypsin is an endogenous enzyme that is used to digest food proteins. That is why it is always secreted by the pancreas shortly after eating. However, the enzyme can also be obtained from animal sources and used medicinally. The protein-splitting effect can, among other things, be used to break down the body's own protein complexes. This is how immune complexes can be resolved in autoimmune diseases.
Inflammation in the musculoskeletal system can also be treated well with trypsin. It also activates the enzyme plasmin from plasminogen. Plasmin dissolves fibrin when there is strong thrombus formation. With the help of trypsin, thrombosis can be treated or even prevented. Furthermore, trypsin supports digestion when taken during meals. When applied 1 to 2 hours before or after a meal, it unfolds its anti-inflammatory effects.
Diseases & Disorders
In the context of pancreatic insufficiency, the synthesis of digestive enzymes such as trypsin can be restricted. The consequence is the development of indigestion. In addition to proteases, the pancreas also produces lipases and amylases.
If the enzymes are missing, the food components are no longer digested and end up in the colon. For example, if there is a lack of trypsin, the protein in the diet can no longer be properly digested. Putrefactive bacteria settle in the large intestine and break down the proteins anaerobically. There are massive digestive problems with flatulence, diarrhea and abdominal pain. Furthermore, the reduced formation of amino acids leads to a protein deficiency and malnutrition despite sufficient food intake. However, the enzymes can also be supplied from outside.
However, there are also medical emergencies in which the body's own enzymes such as trypsin digest the pancreas itself. This can happen if the biliary and pancreatic ducts are blocked. Trypsin is released, but cannot get into the small intestine through the pancreatic blockage. If the pancreatic duct is not opened in this acute emergency situation, the outcome will be fatal due to the self-dissolution of the pancreas. Even with pancreatic tumors, the pancreatic duct can be completely or partially closed. The action of the digestive juices within the pancreas manifests itself as chronic or acute pancreatitis.
A lack of trypsin can also be caused by a mutation. There are also hereditary forms of pancreatitis when the breakdown of trypsin is disturbed.









.jpg)

.jpg)
.jpg)











.jpg)