Aminopenicillins are antibiotics that are used for antimicrobial treatment. Due to the chemical expansion of penicillin with an amino group on the benzyl residue, the drug group shows a broader spectrum of activity than penicillin. Aminopenicillins are used as broad spectrum antibiotics for various bacterial diseases.
What are aminopenicillins?
Aminopenicillin belongs to the group of beta-lactam antibiotics. This is structurally characterized by a four-membered lactam ring, which is formed during biosynthesis. Aminopenicillin and penicillin have the same basic structure. A substituted amino group on the benzyl residue distinguishes the two antibiotics in their chemical structure.
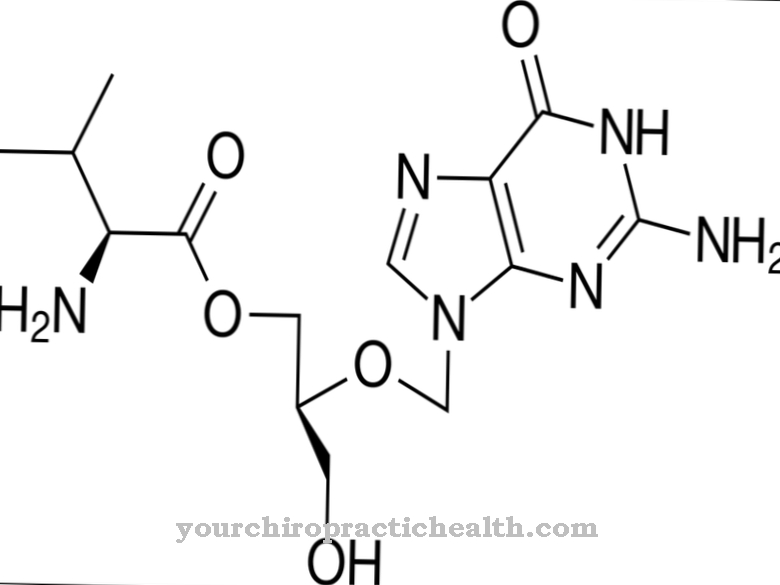
For the production of aminopenicillin an amino group is synthesized at the α-position of the benzylpenicillin. The additional amino group leads to a wider range of action and makes aminopenicillin an effective broad spectrum antibiotic.
ß-lactams (beta-lactams) such as aminopenicillin are acid-resistant and can be administered orally. However, the antibiotic is not resistant to ß-lactamases. ß-lactamases are found in many bacteria and reduce the spectrum of activity of aminopenicillin. ß-lactamase inhibitors prevent the antibiotic from breaking down. In combination with aminopenicillin, ß-lactamase inhibitors increase the spectrum of activity of the antibiotic.
The aminopenicillins include the drugs amoxicillin, ampicillin, pivampicillin and bacampicillin. Pivampicillin and bacampicillin are no longer prescribed. Amoxicillin and ampicillin are also used to treat bacterial diseases.
Pharmacological effect on the body and organs
Aminopenicillin binds proteins via the ß-lactam ring. Like all ß-lactam antibiotics, the ß-lactam ring is the center of action and aminopenicillin binds protein structures identical to penicillin. The protein transpeptidase belongs to the group known as penicillin-binding proteins. The transpeptidase ensures the cross-linking of glycopeptides in a bacterial cell wall. If the enzymes are inactivated by ß-lactam antibiotics, the cross-linking of glycopeptides can no longer take place and the bacterial cell wall becomes unstable. With increasing instability, water flows into the bacterium, builds up an osmotic imbalance and the bacterium bursts.
ß-Lactam antibiotics such as aminopenicillin develop their bactericidal effect on bacteria that proliferate and form a cell wall. Due to the additional amino group on the benzyl residue, aminopenicillins capture more gram-negative bacteria than penicillins. Furthermore, aminopenicillins are four to ten times more effective against gram-negative bacteria compared to penicillins.
The bacterial species detected by aminopenicillins include gram-positive bacteria such as enterococci, listeria, and Streptococcus faecalis. Salmonella, Shigella, Haemophilus influenzae, Escherichia coli, Proteus mirabilis and Helicobacter pylori are gram-negative bacteria that are in the spectrum of activity of aminopenicillins.
While the antibiotic is effective against 60% of the Escherichia coli strains and against most strains of Proteus mirabilis, Haemophilus influenzae strains are often resistant. Bacteria that can produce ß-lactamase are resistant to ß-lactam antibiotics. The spectrum of activity of aminopenicillins is expanded if a ß-lactamase inhibitor such as tazobactam is also taken.
Medical application & use for treatment & prevention
Aminopenicillins are broad spectrum antibiotics and are given in practice for the initial treatment of bacterial infections. A broad spectrum antibiotic is always prescribed for initial treatment when the pathogen is unknown. For the exact and effective use of aminopenicillins it is necessary to create an antibiogram and identify the bacterial strain.
Aminopenicillins are mainly used for respiratory infections, urinary tract infections, sinusitis, otitis media, bacterial endocarditis, listeriosis, epiglottitis, osteomyelitis, meningitis and soft tissue infections.
Treatment of bacterial endocarditis occurs when the patient is infected with enterococci. An aminoglycoside is administered at the same time. Aminopenicillins are only prescribed for urinary tract infections when Proteus mirabilis, enterococci or E. coli trigger the infection.
The bioavailability of an aminopenicillin depends on its chemical structure. The aminopenicillin amoxicillin is preferably administered orally and 60 to 80% is absorbed enterally. The good bioavailability is due to a hydroxyl group substituted on the phenol ring (in the para position). Amoxicillin uses the enteral dipeptide transporter due to the chemical structure change. On the other hand, if the aminopenicillin ampicillin is administered orally, enteral absorption is only 30%. 70% of the active ingredient thus remains in the intestinal lumen. This leads to undesirable side effects in the gastrointestinal area. Furthermore, the plasma level is only insufficiently increased. Ampicillin is preferably administered intravenously (i.v.) or intramuscularly (i.m.) Due to the poor enteral absorption.
Aminopenicillins bind to albumin in the human bloodstream and are excreted renally. Research suggests that a minimal amount of aminopenicillins is metabolised in the liver (hepatically).
Risks & side effects
Gastrointestinal side effects are common after oral ingestion of aminopenicillins. In addition to diarrhea, pseudomembranous enterocolitis can occur. Other side effects are seizures and sensory and motor disorders. These side effects often occur after high doses of antibiotics as a result of neurotoxic reactions and affect the central nervous system.
In the case of infectious mononucleosis (Pfeiffer's glandular fever) or leukemia present at the same time as the infection, macular rashes can occur as a result of the aminopenicillin treatment. A serious side effect with penicillin derivatives such as aminopenicillins is anaphylactic shock.
It is contraindicated in cases of renal insufficiency, chronic lymphocytic leukemia and penicillin allergy.






.jpg)