Desmin is a protein that is found as an intermediate filament in the cell skeleton as well as in striated and smooth muscles. Its task is to stabilize the cells and to connect the muscular structures. Genetic changes (mutations) that cause disorders in desmin synthesis are related to various muscle diseases such as desminopathy or cardiomyopathy.
What is desmin?
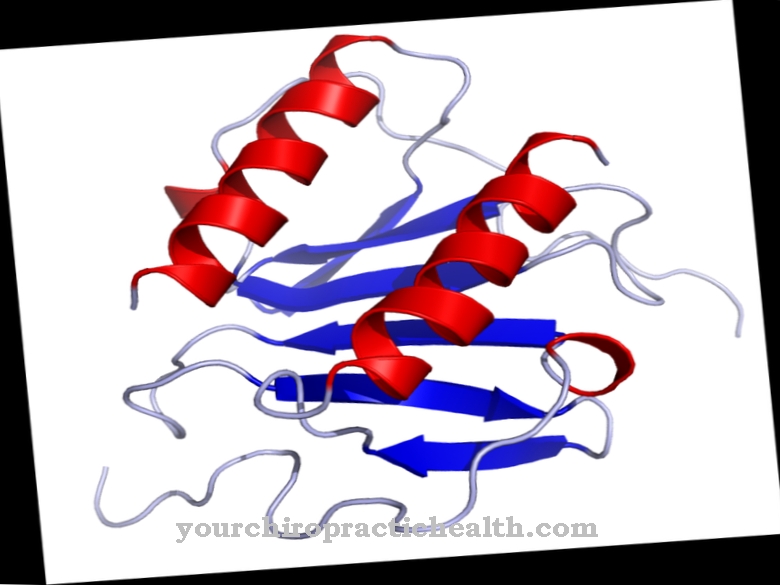
Desmin is a building block of the cell skeleton (cytoskeleton) made of protein. The protein also stabilizes the fine fibrils of the muscles and occurs in striated and smooth muscles. Desmin filaments belong to the intermediate filaments (Filamenta intermedialia), which biology divides into five different types.
According to this classification, desmin belongs to type III together with vimentin, peripherin and glial filament protein (GFAP). Findings from animal experiments suggest that vimentin can possibly replace missing desmin in the early development phases or is able to partially take over its functions. Genes that encode desmin are located in the human genome on the second chromosome in the section 219.99 to 220 Mb. Biology used to refer to desmin as, because of its stabilizing function in the cell skeleton Skeletin. Scientists Lazarides and Hubbard were the first to describe it in 1976.
Anatomy & structure
Like all proteins, desmin is made up of long chains of amino acids. These biological building blocks all follow the same basic structure and only differ from one another in their specific remainder.
Protein synthesis connects individual amino acids with peptide bonds; The sequence of the individual building blocks depends on the sequence that the genes specify through their base sequence. Desmin consists of a total of 470 amino acids. The finished peptide chain represents the primary structure of desmin, which only becomes the finished protein in its spatial shape. After the production of the peptide chain, further connections are formed spontaneously or with the help of enzymes, which are also known as hydrogen bonds and which either arrange the chain into a helix (alpha structure) or format it into a sheet (beta structure).
Desmin consists of longer stretched sections and helix formations. In addition to this secondary structure, the protein assumes a more complex tertiary structure, which is also important for the later function of the protein.In addition, in some cases, various folded amino acid chains combine to form a quaternary structure, within which, in principle, other biomolecules can also occur. Desmin is in the secondary, tertiary and quaternary structure as a homopolymer: The polymer is a structure that consists of several macromolecules. In the case of a homopolymer such as desmin, these macromolecules or monomers are all parts of the same type. A single completed desmin filament has a diameter of 8-11 nm.
Function & tasks
The main task of Desmin is to strengthen the cell skeleton and the muscles, whereby it occurs equally in smooth and striated muscles. In biology, a cell skeleton is a structure within cells that consists of proteins and gives them shape and stability. The cell skeleton also participates in the transport of substances within the cell and in their movements.
In contrast to the bony skeleton of the human body, the cell skeleton does not form a fixed unit, but can flexibly adapt to the needs of the cell. Striated muscles also need desmin as a connecting piece between Z-discs and myofibrils. Z-disks mark the boundaries between adjacent muscle sections (sarcomeres) in the striated muscles. Thread-like structures consisting of a complex of actin and tropomyosin are attached to the Z-disks. When contracted, these myosin fibers and filaments push into one another, causing the tissue as a whole to temporarily shorten.
Smooth muscles have a different structure than striated ones: the fibers do not form clearly delimited threads and bundles with clearly visible stripes in cross-section, but appear smooth and unstructured at first glance. However, the contraction is largely similar. Together with the non-muscular actin filaments of the smooth muscles, desmin also has a stabilizing function in the muscle tissue by creating strengthening connections in so-called compression zones.
Diseases
Various muscle diseases are related to genetic changes (mutations) that affect the desmingencies. In humans, these are on the second chromosome.
Even if such a disease is congenital, it does not have to immediately manifest itself in visible symptoms. In many cases, desmin mutations lead to muscular dystrophy, which is characterized by the progressive deterioration of the muscular tissue. The appearance of the dystrophies is very heterogeneous. Desminopathy is a more specific clinical picture. This is a rare hereditary disease that gradually weakens muscles and typically only leads to symptoms in adulthood. Errors in the body's own production of desmin impair both the cytoskeleton of the muscle cells and the Z-discs in desminopathy.
In addition, desmin mutations are associated with cardiomyopathy, which can also occur in the context of desminopathy. Cardiomyopathy manifests itself in functional heart problems and is not always due to disorders in the desmin synthesis; instead, there are a variety of possible causes. Typical symptoms include cardiac insufficiency, cardiac arrhythmias, circulatory collapse (syncope), angina pectoris and embolisms.
Detection of desmin antibodies also helps doctors differentiate between different tumors - for example rhabdomyosarcomas (malignant tumors in soft tissue with a high mortality rate) and leiomyosarcomas (malignant tumors in smooth muscles).





















.jpg)



