Elastases represent a group of proteases that are closely related to the enzymes trypsin and chymotrypsin. They belong to the serine proteases. So far, nine enzymes belonging to the elastases are known for the human organism.
What are elastases?
Elastases are non-specific proteases that occur in all animal and human organisms. The name comes from the fact that, among other things, they are able to break down the body's own elastin. The elastases belong to the serine proteases.
Their active center contains the so-called catalytic triad of aspartic acid, serine and histidine. Furthermore, the elastases also belong to the endoproteases. They do not break down the proteins and polypeptide chains step by step, but split them at certain amino acids and characteristic amino acid sequences. The protein is broken down within the peptide chain. The effect of the elastases is not specific. In this way, the body's own proteins can also be broken down from elastin. Therefore, the effect of these enzymes must be limited by elastase inhibitors. A distinction is made between two types of elastases.
There are pancreatic elastases and granulocyte elastases. As the name suggests, pancreatic elastases (elastase 1) are secreted from the pancreas. Granulocyte elastase (elastase 2) is located in the neutrophil granulocytes. A deficiency of elastase 1 in the stool is considered evidence of pancreatic insufficiency.
Function, effect & tasks
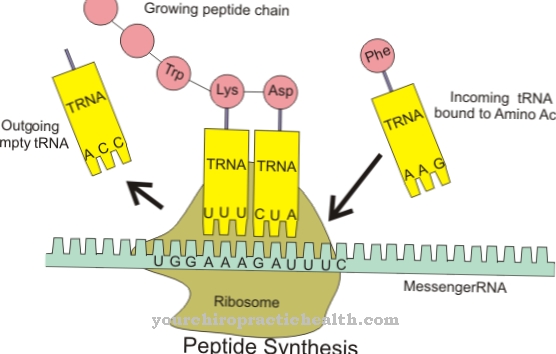
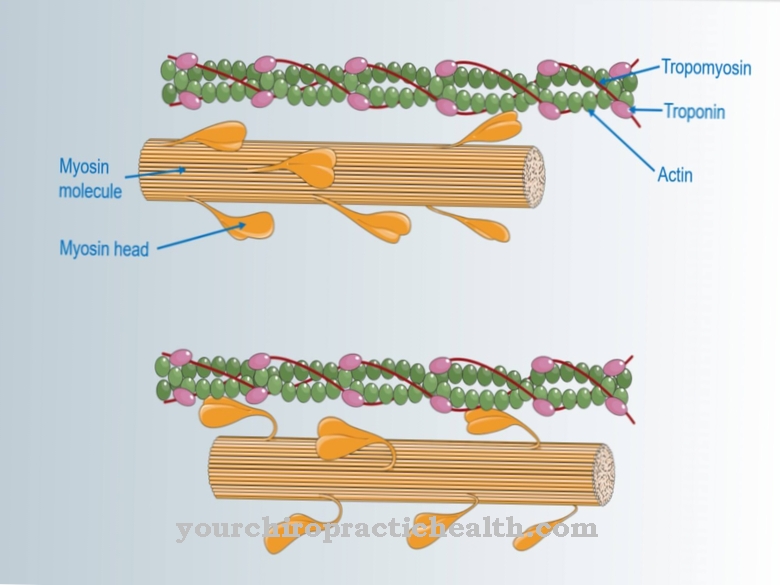
Elastases have the task of breaking peptide bonds in proteins or polypeptide chains. Smaller peptide chains or individual amino acids are formed in the process. The pancreatic elastase supports the proteases trypsin and chymotrypsin in breaking down dietary proteins.
It is formed in the pancreas as an inactive proenzyme (zymogen) and, after being released in the small intestine, is converted into the active form by the action of trypsin. A partial chain is split off from the zymogen. Elastase 1 breaks down the fiber protein elastin in particular. Elastin is part of the connective tissue of the lungs, blood vessels and skin. It mainly has a supporting function in the organism. Elastin gives the organs shape and support. Since it forms protein networks through the aggregation of four lysine molecules, it cannot be broken down by many proteases. However, Elastase 1 has the ability to do so. Elastin components from food are broken down and can be processed further and broken down into amino acids.
Unfortunately, the effect of elastase is unspecific, so that it can also attack the body's own elastin structures. To do this, the body produces elastin inhibiting proteins that can control the destructive effects of elastin. These proteins include α1-antitrypsin, alpha-2-macroglobulin or elafin. The second group of elastases is represented as ELA-2, the granulocyte elastase. Their job is to break down phagocytosed microorganisms as part of an immune response to infections. However, they also have a non-specific effect and attack the body's own elastin. If the effect of the elastase inhibitor proteins is restricted, lung tissue can be destroyed, with the formation of emphysema.
Education, occurrence, properties & optimal values
Regardless of where they are synthesized, elastases are important supporters of the immune system in the fight against gram-negative germs in the digestive tract, in the lungs and on wounds. In doing so, they cleave the corresponding proteins on the carboxy side of hydrophobic amino acids, including valine, glycine and alanine. However, as already mentioned, their effect is always unspecific.
The human body uses around 500 milligrams of elastase every day. Elastase is not broken down in the body. It is excreted unchanged in the stool. The function of the pancreas can be checked using the amount excreted in the stool. Chymotrypsin is also excreted with the faeces. However, the determination of elastase can be used more clearly for diagnostic purposes. The normal concentration of elastase is at least 200 micrograms per gram of stool.
Diseases & Disorders
If the level of elastase in the stool is too low, this indicates pancreatic insufficiency. If the value is between 100 and 200 micrograms per gram of faeces, it is a matter of a mild to moderate dysfunction of the pancreas.
Severe pancreatic insufficiency is present at values below 100 micrograms. The detection of elastase in the stool is a characteristic diagnostic feature of an underactive pancreas. This is the exocrine function of the pancreas. The formation of insulin can be unaffected. In the case of pancreatic insufficiency, too few digestive enzymes are secreted. This applies to proteases as well as lipases and amylases. Many food components reach the large intestine undigested, where they are further broken down by pathogenic bacteria. The pathogenic germs can only thrive if there are still enough undigested food components. Putrefaction and fermentation processes develop, which lead to meteorism, diarrhea and abdominal discomfort.
Since fats are no longer broken down, fatty stools can occur. The cause of the underactive pancreas can be due to acute or chronic pancreatitis. Pancreatitis usually results from the self-digestion of part of the pancreas by non-draining digestive juices. The pancreatic outlet can be narrowed due to tumors or gallstones. Drainage disorders due to malformations are also possible. Long-term chronic pancreatitis leads to impaired function of the pancreas with reduced enzyme production. If there is an elastase deficiency of elastase 2 due to a genetic defect, the immune system of the affected patient is weakened. Life-threatening infections are constantly occurring.
If there is a lack of elastase inhibitors such as alpha-1-antitrypsin or increased activity of elastase in pneumonia, the lung function can be severely restricted. In the long term, pulmonary emphysema develops from this. In the case of genetic alpha-1-antitrypsin deficiency, life-long substitution therapy with genetically engineered alpha-1-antitrypsin is used.









.jpg)




.jpg)