Homocysteine is a non-proteinogenic sulfur-containing alpha-amino acid, which is formed as an intermediate from methionine by releasing the methyl group (-CH3).
For the further processing of homocysteine, an adequate supply of vitamins B12 and B6 as well as folic acid or betaine as a supplier of methyl groups is necessary. An increased concentration of homocysteine in the blood plasma is associated with damage to the blood vessel walls, dementia and depression.
What is homocysteine?
Homocysteine in its bioactive L-form is a non-proteinogenic amino acid. It cannot be a building block of a protein because it tends to form a heterocyclic ring that does not allow a stable peptide bond because of its additional CH2 group compared to cysteine.
The incorporation of homocysteine into a protein would therefore cause the protein to break down soon. The chemical formula C4H9NO2S shows that the amino acid consists exclusively of substances that are available in abundance almost everywhere. Trace elements, rare minerals and metals are not required for their structure. Homocysteine is a zwitterion because it has two functional groups, each with a positive and a negative charge, which overall are electrically balanced.
At room temperature, homocysteine is a crystalline solid with a melting point of around 230 to 232 degrees Celsius. The body can break down an increased homocysteine level in the blood by two homocysteine molecules forming a disulphide bridge to form homocystine, which can then be excreted by the kidneys.
Function, effect & tasks
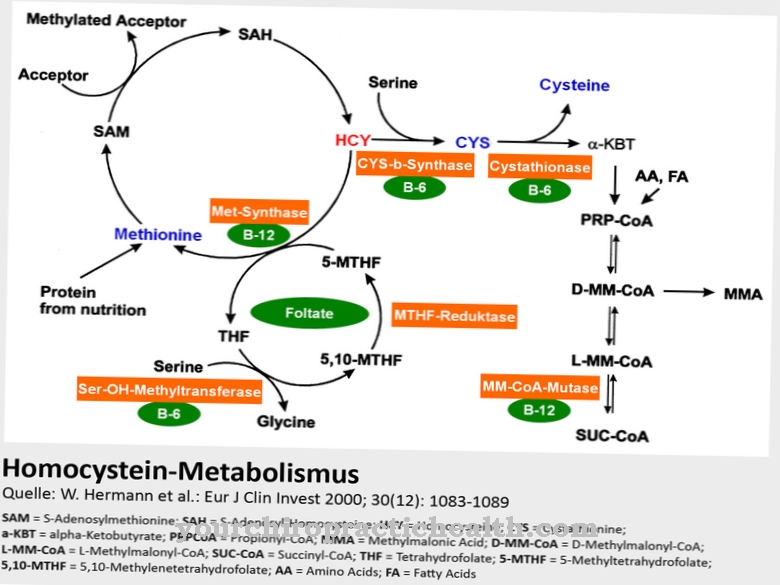
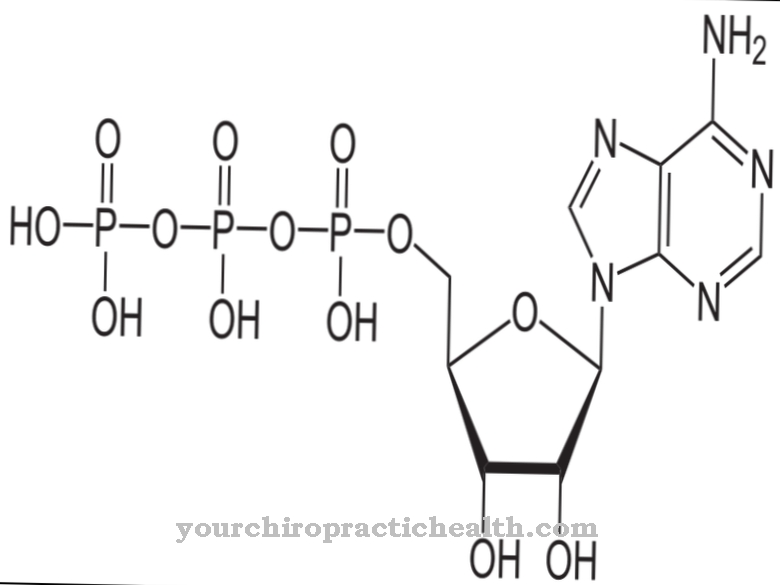
The most important task and function of the L-homocysteine is to support the synthesis of proteins and to be converted into S-adenosylmethionine (SAM) in cooperation with some co-enzymes. With three methyl groups (-CH3), SAM is the most important methyl group donor in cellular metabolism.
SAM is involved in many biosynthesis and detoxification reactions. The methyl groups of certain neurotransmitters like adrenaline, choline and creatine come from the SAM. After a methyl group has been released, SAM produces S-adenosylmethionine (SAH), which is converted back to adenosine or L-homocysteine again by hydrolysis. As important as homocysteine's supporting function for certain metabolic processes is, it is also important that homocysteine, as an intermediate product of these biochemical reaction and synthesis chains, does not occur in abnormal concentrations in the blood because it then develops harmful effects.
Excess homocysteine, which is not required to support the above-described reactions in the methionine metabolism, is therefore normally further broken down with the participation of vitamin B6 (pyridoxine) and excreted via the kidneys after the formation of homocystine. In order for homocysteine to perform its metabolic tasks, it is important to supply the body with sufficient amounts of vitamins B6, B12 and folic acid.
Education, occurrence, properties & optimal values
Homocysteine is produced in the body as a short-lived intermediate product within the complex methionine metabolism. The alternative designation (S) -2-amino-4-mercaptobutanoic acid indicates the structure of homocysteine. It is therefore a monocarboxylic acid with the characteristic carboxy group (-COOH) and at the same time a simple fatty acid. Homocysteine is not absorbed through food, but only temporarily produced in the body.
Although the bioactive L-cysteine plays an important role in protein synthesis and in the formation of SAM, the optimal and at the same time tolerable concentration in the blood is within narrow limits of only 5 to 10 µmol / liter. Higher homocysteine levels indicate certain metabolic disorders and lead to the clinical picture of hyperhomocysteinemia. An optimal concentration of the amino acid is likely to depend on the respective mental and physical activity and is difficult to define. The definition of a tolerable upper limit for the homocysteine level, which should be around 10 µmol / liter, appears more sensible.
Diseases & Disorders
If the concentration of homocysteine exceeds the tolerable limit, there are mostly acquired or genetically determined metabolic disorders in the methionine balance.
Often there is only a lack of the necessary vitamins B6 (pyridoxine), B9 (folic acid) and B12 (cobalamin), which are required as coenzymes or catalysts within the biochemical conversion chain. A total of about 230 - albeit rarely occurring - gene mutations that lead to a disruption of the methionine metabolism are known. The pathological increase in homocysteine is called homocystinuria. The most common gene mutation that causes the disease is located on gene locus 21q22.3. The mutation is autosomal recessive and causes the formation of a defective enzyme that is required for the process of breaking down and converting homocysteine.
The previously known mutations are the omission (deletion) or the addition (insertion) of nucleobases on the corresponding DNA strands. Unfavorable living conditions and habits can also cause increased homocysteine levels. These include excessive alcohol consumption, nicotine abuse, overweight and sedentary lifestyle. An excessive homocysteine level can damage the endothelium, the inner wall of the blood vessels, and B. Promote arteriosclerosis. The veins become inelastic and cause a number of secondary diseases such as high blood pressure. They also harbor the risk of forming thrombi, which cause coronary heart disease and strokes.
Neurological diseases such as depression and senile dementia are also associated with an increased homocysteine level. The symptoms of the disease are very different in children who suffer from genetic homocystinuria. The spectrum of symptoms ranges from barely detectable disease characteristics to the appearance of almost all possible symptoms. The first symptoms usually only appear after reaching the age of two. At most, a slowdown in psychomotor development can be seen during the first two years of life. In many cases, the first symptom of genetic homocystinuria is a prolapse of the lens of the eye.

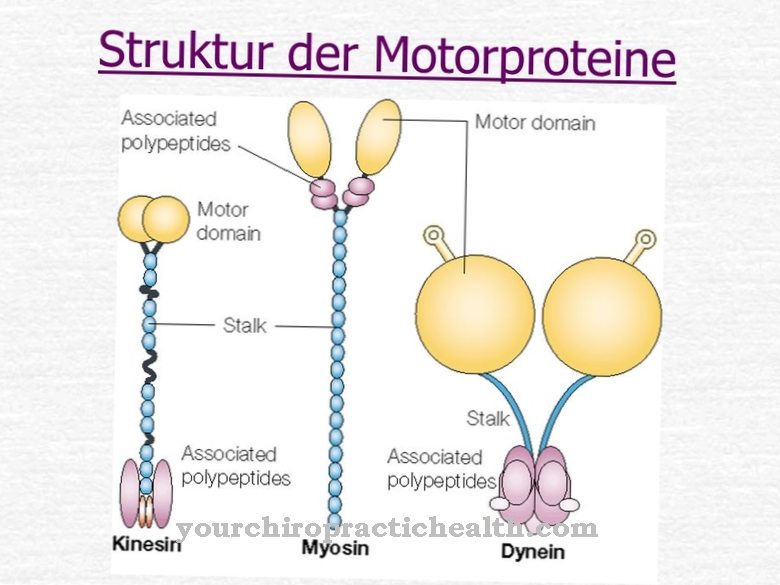



















.jpg)



