Transthyretin is a transport protein for thyroid hormones found in all vertebrates. It is synthesized in the liver and in certain areas of the brain. Specific genetic changes in transthyretin can lead to amyloidosis type 1 or ATTR amyloidosis.
What is transthyretin?
Transthyretin (TTR) belongs to the transport proteins. In addition to the thyroxine-binding globulin (TBG), it is also a protein that binds thyroid hormones. However, its binding force is not as strong as that of TBG.
L-thyroxine (T4) is 99.99 percent and triiodothyronine (T3) at least 99 percent bound to TBG. Transthyretin binds to the thyroid hormone T4 with a lower affinity. There is no binding at T3. Linking to the transport proteins significantly increases the half-life of the thyroid hormones in the body, because this significantly delays their excretion in the urine. The half-life for T4 is around five to eight days. At T3, however, it is only about 19 hours because its binding to TBG is much lower and it is not bound to transthyretin at all.
The total concentration of thyroid hormones depends on the concentration of the transport proteins. However, unlike the free thyroid hormones, the bound thyroid hormones are not biologically active. The main sites for transthyretin are the liver and choroid plexus. The choroid plexus is a ball-like arteriovenous vessel structure in the cerebral ventricles.
Anatomy & structure
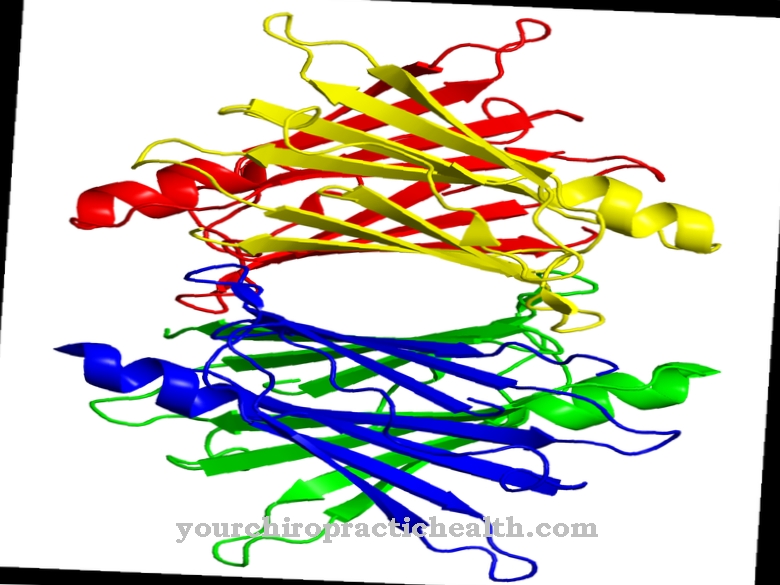
Transthyretin is a protein molecule that consists of 127 amino acids. Its secondary, tertiary and quaternary structure is composed of homotetramers. The transport protein can be determined by means of serum electrophoresis. It appears before the albumin peak, which is why transthyretin is also the alternative name Prealbumin brought in.
Transthyretin has a molar mass of 55 kDa. The chemical structure of transthyretin allows it to bind to the thyroid hormones and to retinol. Since its concentration drops in chronic inflammatory reactions, it is also known as anti-acute phase protein. Acute phase proteins are produced to a large extent in inflammatory reactions. In this way, they are quickly available to the body in the event of necessary defense reactions. The opposite is true for anti-acute phase proteins such as transthyretin.
Function & tasks
The main function of transthyretin is to bind to and transport thyroid hormones. Together with TBG, it ensures its longer half-life in the organism. The thyroid hormones are inactive when bound, but they can be released at any time if necessary.
Another function of transthyretin is to bind to retinol. It forms complexes with the retinol-binding protein. Retinol is free vitamin A, which takes on a variety of tasks in the body. It is responsible for the visual process and participates in the function of the skin, mucous membranes, the immune system, metabolism and blood cells. Both thyroid hormones and retinol are only active in the free form. However, their binding to transport molecules such as transthyretin prevents uncontrolled reactions of these active substances. The controlled release from the bond with the transport proteins ensures that these substances work properly.
Diseases
Various mutations of transthyretin can cause its deficiency as well as a stronger bond to the thyroid hormones (hyperthyroxinemia). In hyperthyroxinemia, the total thyroid gland values are increased. But the concentration of free thyroid hormones is normal.
Accordingly, normal thyroid function takes place. There are no symptoms. Hyperthyroxinemia is sometimes confused with hyperthyroidism (overactive thyroid gland). The difference, however, is that hyperthyroidism has both an increased total thyroid concentration and a higher concentration of free thyroid hormones. In connection with transthyretin, however, type 1 amyloidosis (TTR amyloidosis) often occurs. It is often the result of a lack of transthyretin, which in turn is genetic.
In amyloidosis, small protein fibers that are no longer soluble are deposited in the spaces between the cells, the interstitium. These fibers are in the form of so-called beta fibrils called amyloid. Amyloidosis is not an independent disease, but is a collective term for several different diseases with pathological deposition processes. Depending on the cause, certain organs are affected by the deposition of defective protein fibers. TTR amyloidosis caused by transthyretin may involve the heart, nervous system, intestines, eyes, lungs or kidneys, among others.
Cardiac insufficiency with cardiac arrhythmias, sensory disturbances in the hands and feet, diarrhea, constipation, weight loss or, in rare cases, severe kidney damage up to and including dialysis is possible. Because transthyretin is produced in the liver, a liver transplant can cure this form of amyloidosis. The new healthy liver synthesizes normal transthyretin again. The deposition process comes to a standstill. If the disease is more advanced, a liver transplant cannot guarantee a cure. A special form of TTR amyloidosis is ATTR amyloidosis (senile amyloidosis). This form of the disease occurs particularly in old age. Here, too, the cause is to be found in genetic changes in transthyretin.
If left untreated, amyloidosis leads to death within a few years. In addition to a causal liver transplant, symptomatic therapies must be carried out. These depend on which organs are particularly affected. If the heart is involved, diuretics and ACE inhibitors are administered. If cardiac arrhythmias occur, a pacemaker can help. It is important to eat a low-salt diet. If the kidneys are involved, a low-salt diet, ACE inhibitors and diuretics are also indicated. Dialysis may be necessary.


.jpg)








.jpg)

.jpg)
.jpg)











.jpg)